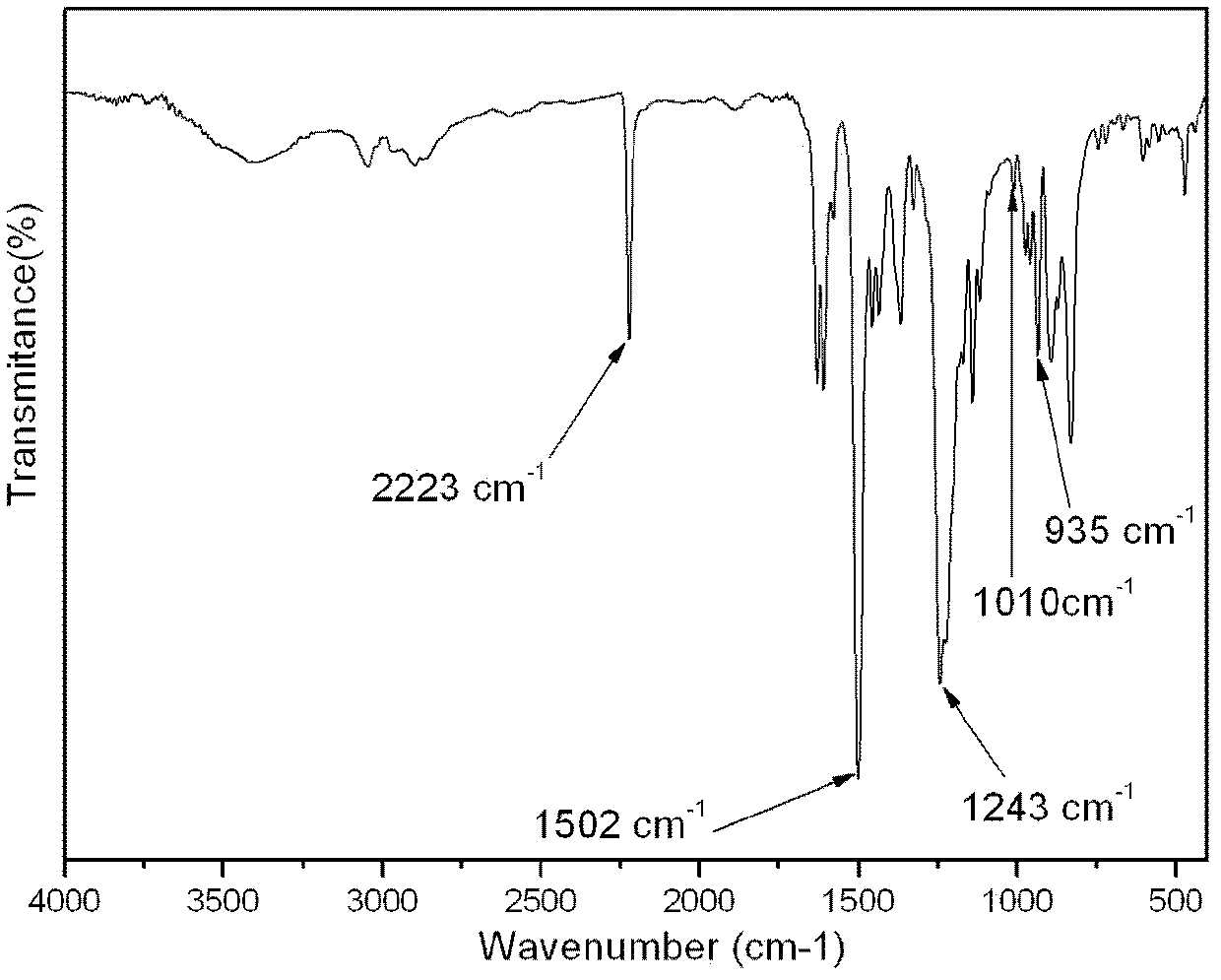
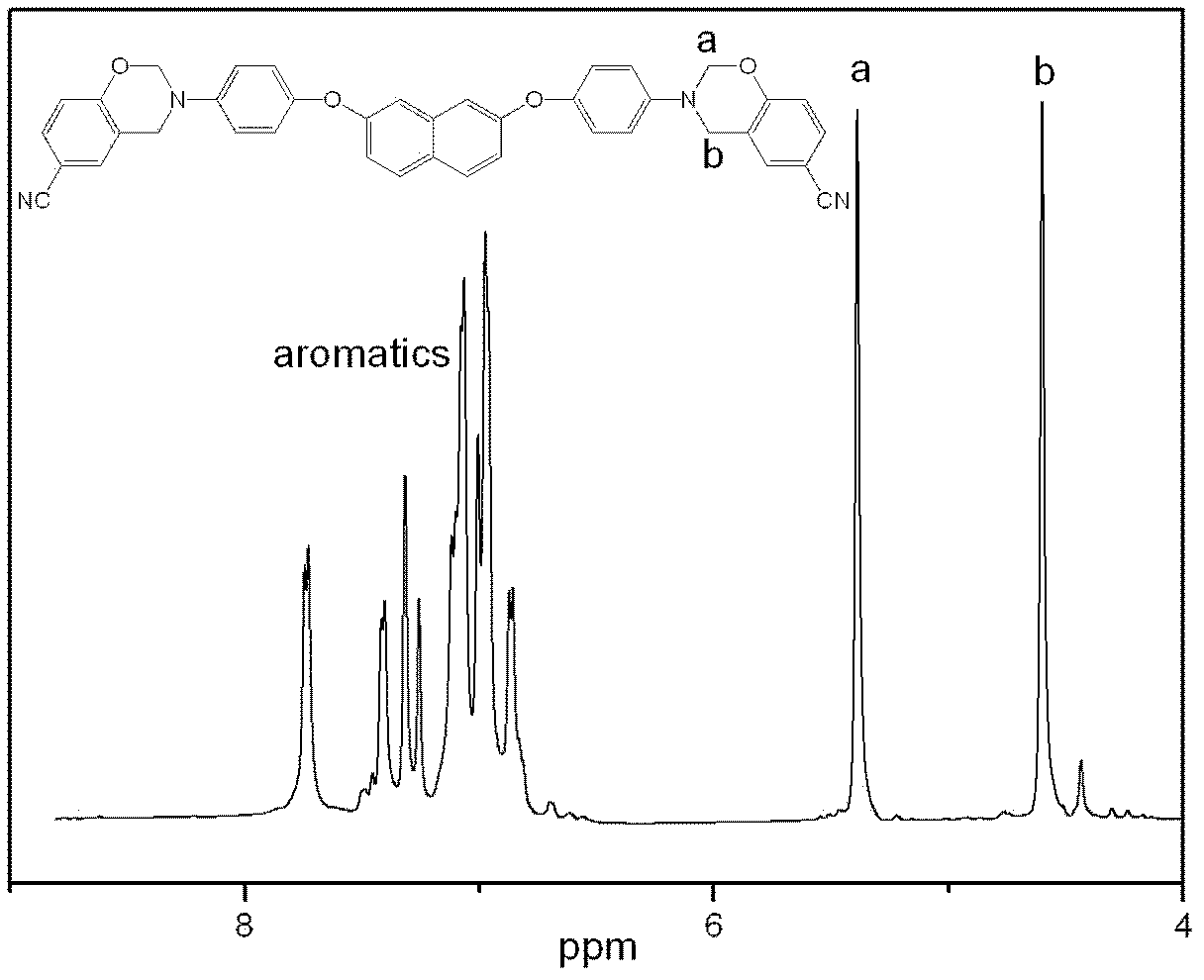
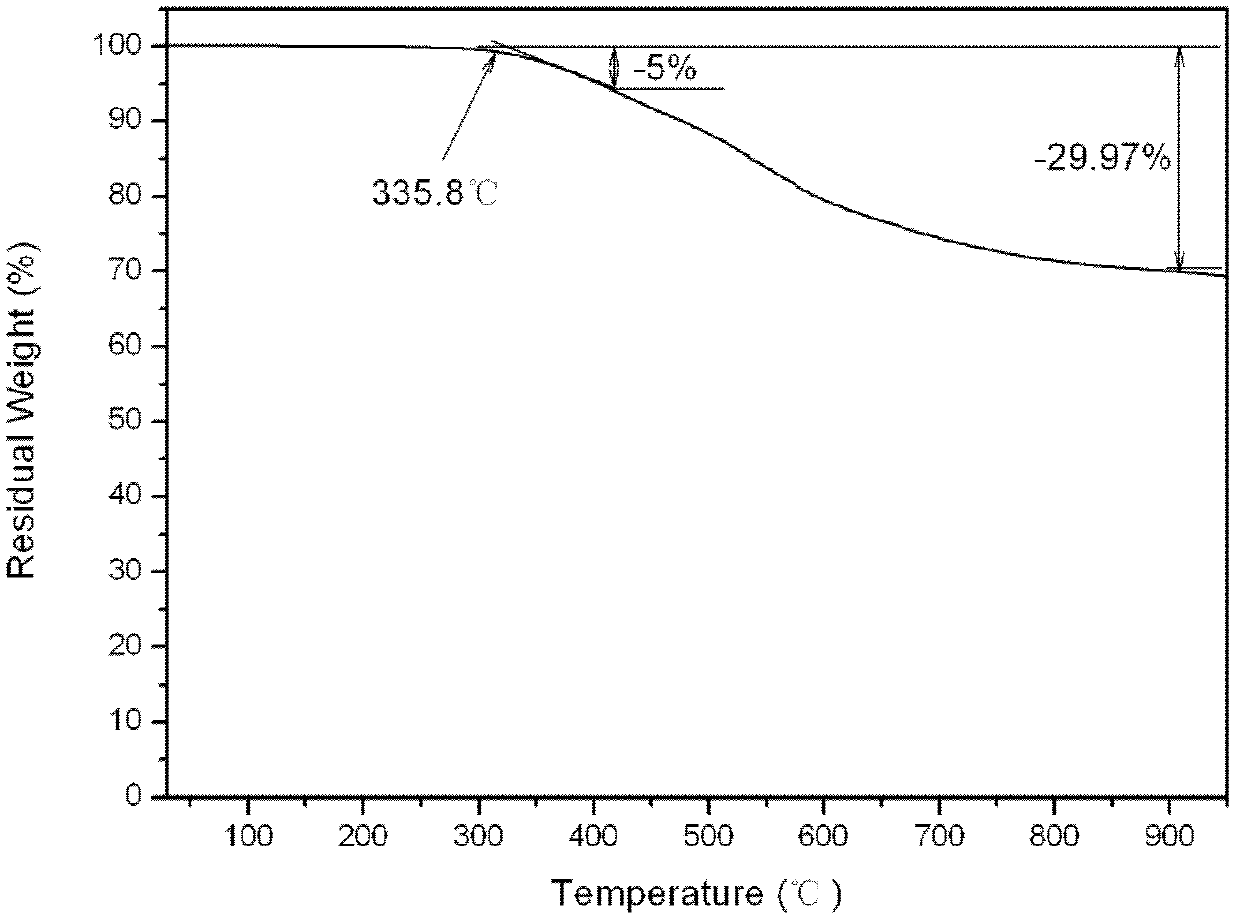
Aromatic diamine type cyano group-containing benzoxazine resin and its preparation method
A technology of aromatic diamine type and oxazine resin, which is applied in the field of thermosetting resin and its preparation, can solve the problems of high brittleness, unsatisfactory process performance, high curing temperature, etc., and achieve the effect of low cost, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a four-necked flask equipped with magnetic stirrer, thermometer, nitrogen pipe, water separator and spherical condenser, add 0.2mol 2,7-dihydroxynaphthalene, 0.44mol p-nitrochlorobenzene, 0.24mol potassium carbonate in turn , 60ml of toluene and 240ml of N,N-dimethylformamide; under the protection of nitrogen, the temperature was slowly raised to 145℃ and reacted at a constant temperature for 8 hours; after the reaction, the inorganic salt was filtered while hot, concentrated by rotary evaporation, and deionized water was added gradually. After cooling down to room temperature, the product slowly precipitated out; the crude product was collected by filtration and dried in vacuum for 24 hours (85°C) to obtain a dihydroxynaphthalene type aromatic dinitro compound with a yield of 99%.
[0032] In a four-necked flask equipped with a magnetic stirrer, thermometer, nitrogen pipe and spherical condenser, put 0.0375mol dihydroxynaphthalene type aromatic dinitro compound, 0.125g ...
Embodiment 2
[0036] In a four-necked flask equipped with a magnetic stirrer, thermometer, nitrogen pipe, water separator and spherical condenser, add 0.2mol 2,7-dihydroxynaphthalene, 0.4mol p-nitrochlorobenzene, 0.2mol potassium carbonate in sequence , 50ml of toluene and 240ml of N,N-dimethylformamide; under the protection of nitrogen, the temperature was slowly increased to 145℃, and the reaction was kept at a constant temperature for 8 hours; after the reaction, the inorganic salt was filtered while hot, concentrated by rotary evaporation, and deionized water was added gradually. The product was cooled to room temperature to slowly precipitate out; the crude product was collected by filtration, and dried in vacuum for 24 hours (85°C) to obtain a dihydroxynaphthalene type aromatic dinitro compound with a yield of 90%.
[0037] In a four-necked flask equipped with a magnetic stirrer, thermometer, nitrogen pipe and spherical condenser, put 0.015mol dihydroxynaphthalene type aromatic dinitro co...
Embodiment 3
[0041] In a four-necked flask equipped with a magnetic stirrer, thermometer, nitrogen pipe, water separator and spherical condenser, add 0.2mol 2,7-dihydroxynaphthalene, 0.42mol p-nitrochlorobenzene, 0.21mol potassium carbonate in sequence , 50ml of toluene and 240ml of N,N-dimethylformamide; under the protection of nitrogen, the temperature was slowly increased to 145℃, and the reaction was kept at a constant temperature for 8 hours; after the reaction, the inorganic salt was filtered while hot, concentrated by rotary evaporation, and deionized water was added gradually After cooling down to room temperature, the product was slowly precipitated out; the crude product was collected by filtration and dried in vacuum for 24 hours (85°C) to obtain a dihydroxynaphthalene type aromatic dinitro compound with a yield of 92%.
[0042] In a four-necked flask equipped with a magnetic stirrer, thermometer, nitrogen pipe and spherical condenser, put 0.015mol dihydroxynaphthalene type aromatic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com