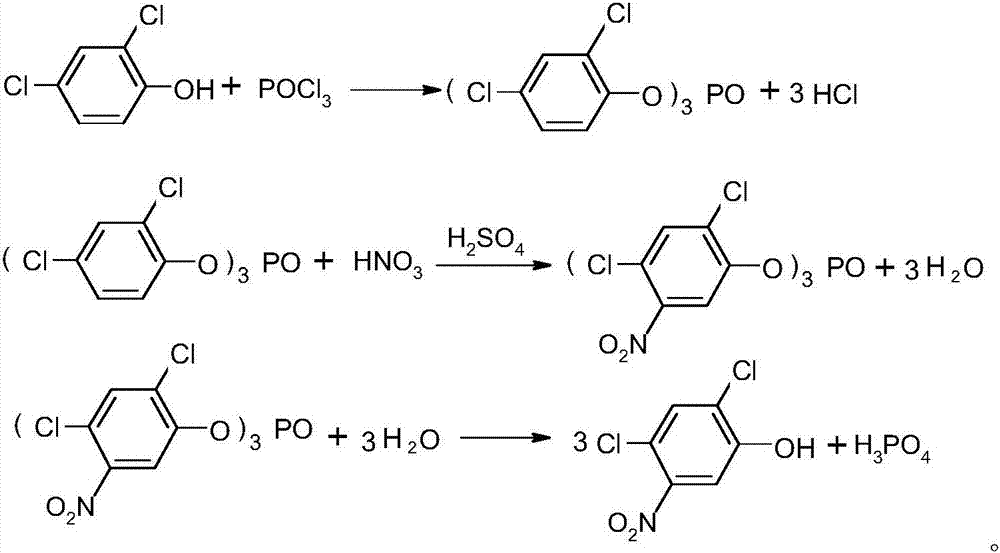
A kind of preparation method of 2,4-dichloro-5-nitrophenol
A technology of nitrophenol and nitrophenyl, which is applied in the field of organic chemistry, can solve the problems of low yield and pollution of the environment by waste acid, and achieve the effects of reducing production costs, reasonable synthesis process, and avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
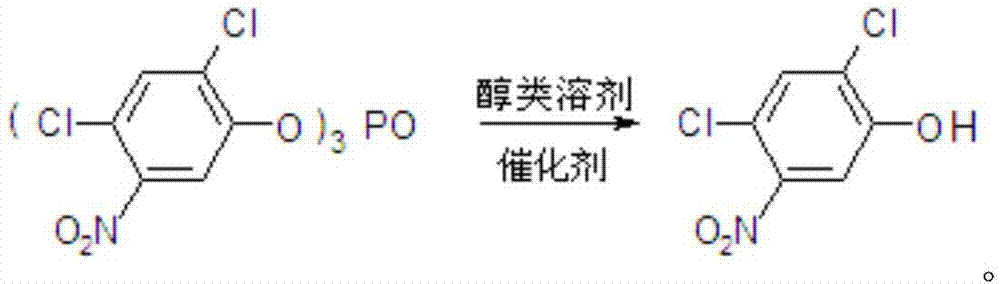
[0027] In a 1000mL four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 96g methanol, 57.8g tris(2,4-dichloro-5-nitrophenyl) phosphate and 0.586g catalyst tri Lanthanum fluoromethanesulfonate, 0.72g tetrahydrofuran, stirred and gradually raised to reflux temperature, reacted for 3h, after the reaction was completed, the catalyst was separated by filtration, and after the solvent was removed, 62.7g of 2,4-dichloro-5-nitrophenol was obtained , content 98.0%, yield 98.5%.
Embodiment 2
[0029] In a 1000mL four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 192g methanol, 57.8g tris(2,4-dichloro-5-nitrophenyl) phosphate and 2.93g catalyst tri Lanthanum fluoromethanesulfonate, 1.8g tetrahydrofuran, stirred and gradually heated to reflux temperature, reacted for 5h, after the reaction was completed, the catalyst was separated by filtration, and after the solvent was removed, 62.8g of 2,4-dichloro-5-nitrophenol was obtained , content 98.3%, yield 99.0%.
Embodiment 3
[0031] In a 1000mL four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser tube, add 288g methanol, 57.8g tris(2,4-dichloro-5-nitrophenyl) phosphate and 5.86g catalyst tri Lanthanum fluoromethanesulfonate, 3.6g tetrahydrofuran, stirred and gradually heated to reflux temperature, reacted for 7 hours, after the reaction was completed, the catalyst was separated by filtration, and after the solvent was removed, 63.2g of 2,4-dichloro-5-nitrophenol was obtained , content 98.5%, yield 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com