Method for recovering cadmium from copper cadmium residues
A technology for copper cadmium slag and copper liquid is applied in the field of cadmium recovery, which can solve the problems of large consumption of zinc powder, complicated recovery process of cadmium, low recovery rate of cadmium, etc., so as to reduce the consumption of zinc powder, the steps of zinc powder replacement and the consumption of zinc powder. volume reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
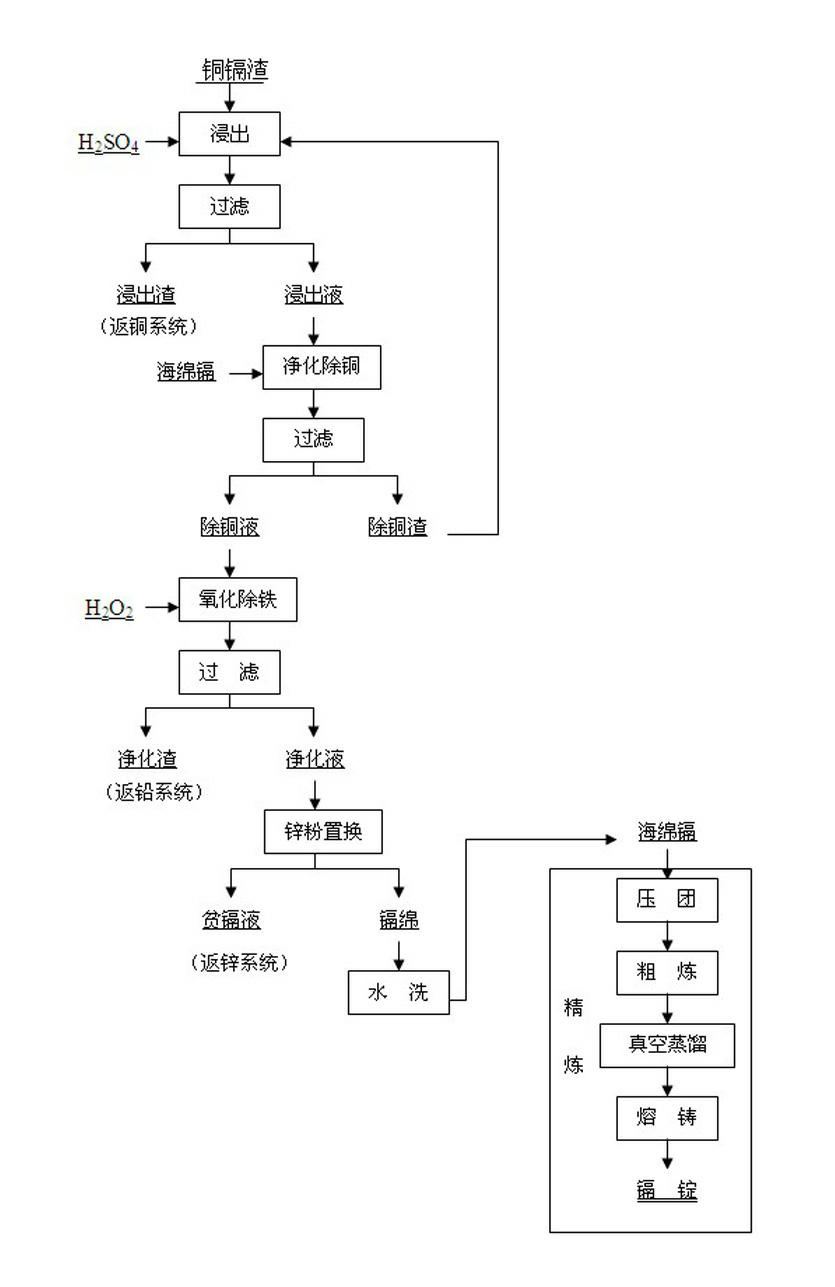
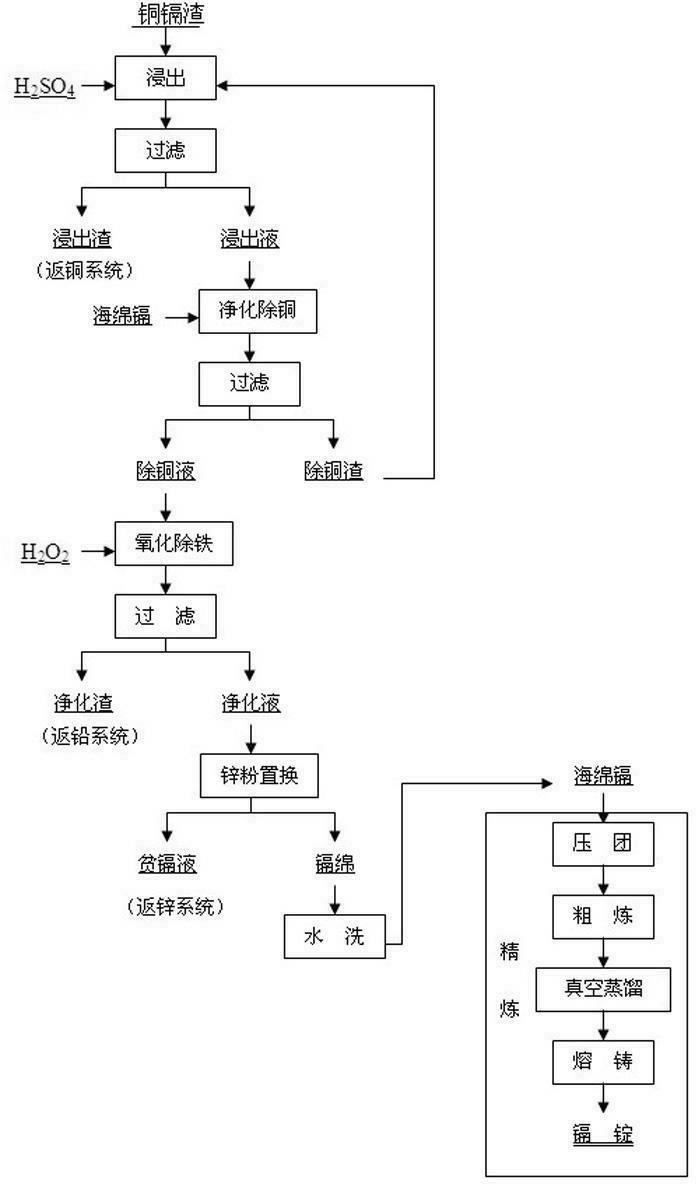
[0054] (1) Sulfuric acid leaching: immerse copper cadmium residue in sulfuric acid at 85°C, the concentration of sulfuric acid is 8g / L, and filter to obtain leachate. The components of the leachate are: Cd 21.55g / L, Zn 170.97g / L, Fe 1.43g / L, Cu 0.081g / L, Sn 0.0073g / L, Pb 0.096g / L, As 0.010g / L, Sb 0.0064g / L, endpoint pH=2.5.
[0055] (2) Purify and remove copper: raise the temperature of the leaching solution in step (1) to 52°C, add 310g of fresh cadmium sponge (1.5 times the theoretical consumption) under stirring; check the pH of the solution during the addition process, when the pH is low, Appropriately add fresh cadmium cotton, control the end point pH=3.6, and obtain the copper removal solution after filtration.
[0056] (3) Oxidative iron removal: heat up the copper removal solution in step (2) to 58°C, add 31L of hydrogen peroxide (1.3 times the theoretical consumption) under stirring, take a sample for As and Sb after a reaction time of 30 minutes, and filter it after ...
Embodiment 2
[0062] (1) Sulfuric acid leaching: soak copper cadmium slag in sulfuric acid at 90°C. The acidity of sulfuric acid is 10g / L. After filtration, the leachate is obtained. The components of the leachate are: Cd 24.38g / L, Zn 182.11g / L, Fe 1.98g / L, Cu 0.095g / L, Sn 0.0054g / L, Pb 0.088g / L, As 0.009g / L, Sb 0.0072g / L, endpoint pH=3.0.
[0063] (2) Purify and remove copper: raise the temperature of the leaching solution in step (1) to 58°C, add 436g of fresh cadmium sponge (1.8 times the theoretical consumption) under stirring; check the pH of the solution during the addition process, when the pH is low, Appropriately add fresh cadmium cotton, control the end point pH=3.2, and obtain the copper removal solution after filtration.
[0064] (3) Oxidative iron removal: raise the temperature of the copper removal solution in step (2) to 57°C, add 46L hydrogen peroxide (1.4 times the theoretical consumption) under stirring, take a sample after 24 minutes to test As and Sb, and filter it after...
Embodiment 3
[0070] (1) Sulfuric acid leaching: soak copper cadmium slag in sulfuric acid at 95°C. The acidity of sulfuric acid is 10g / L. After filtration, the leachate is obtained. The components of the leachate are: Cd 26.71 g / L, Zn 187.58 g / L, Fe 2.26g / L, Cu 0.12g / L, Sn 0.011g / L, Pb 0.084g / L, As 0.059g / L, Sb 0.011g / L, pH=2.8.
[0071] (2) Purify and remove copper: heat the leaching solution in step (1) to 55°C, add 612g of fresh cadmium sponge (2.0 times the theoretical consumption) under stirring; check the pH of the solution during the addition process, when the pH is low, Appropriately add fresh cadmium cotton, control the end point pH=3.4, and obtain the copper removal solution after filtration.
[0072] (3) Oxidative iron removal: heat up the copper removal solution in step (2) to 60°C, add 56L hydrogen peroxide (1.5 times the theoretical consumption) under stirring, take a sample after 20 minutes of reaction time to test As and Sb, and filter it after passing the test. Iron remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com