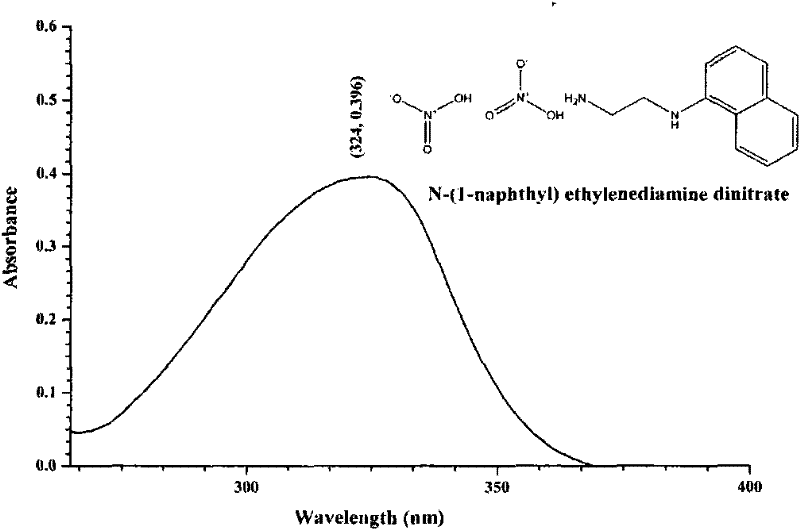
N-(1-naphthyl) ethylenediamine dinitrate and preparation method and application thereof
A technology of naphthaleneethylenediamine nitrate and naphthaleneethylenediamine hydrochloride, which is applied in the fields of organic synthesis and mass spectrometry detection, and can solve the problems of long time consumption, poor repeated use effect, and decline in analysis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1, naphthalene ethylenediamine nitrate
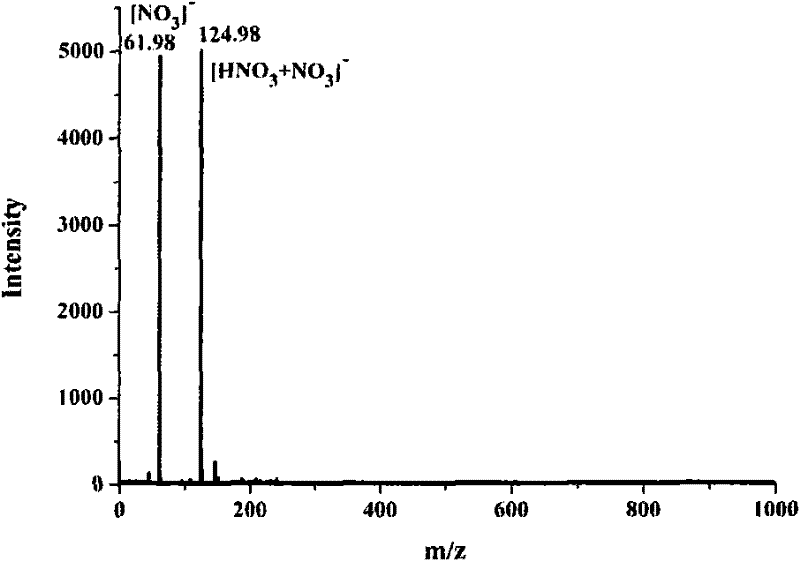
[0032] At room temperature, 33.50mg of naphthaleneethylenediamine hydrochloride and 44.71mg of AgNO 3 (Naphthylethylenediamine hydrochloride and AgNO 3 The molar ratio is 1:2) to 1.200ml of ethanol / water with a volume ratio of 30:70, so that the two are mixed uniformly by ultrasound, and reacted; during the reaction, white AgCl precipitates will be produced; when the white AgCl When the precipitation no longer increases, it indicates that the reaction is complete, the supernatant liquid of the upper layer is the ethanol / water solution of naphthalene ethylenediamine nitrate, and the precipitate of the lower layer is AgCl. After separating and filtering out the precipitate AgCl, the ethanol / water solution of naphthaleneethylenediamine nitrate in the upper layer is passed through 732 type strongly acidic cation exchange resin, and the effluent is pure ethanol / water solution of naphthaleneethylenediamin...
Embodiment 2
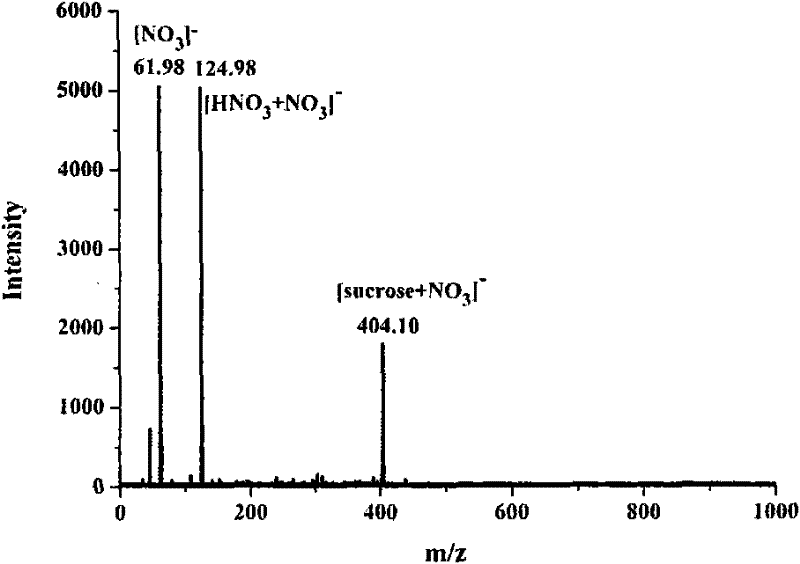
[0036] Embodiment 2, the molecular weight of sucrose is measured as matrix with the naphthalene ethylenediamine nitrate prepared in embodiment 1
[0037](1) Dissolving 3.41 mg of sucrose with 1.000 ml of methanol / water solution with a volume ratio of 50:50 to obtain methanol / water solution of sucrose, the molar concentration of the solution is 10 mmol / L;
[0038] (2) directly use the ethanol / water solution of the naphthalene ethylenediamine nitrate prepared by embodiment 1, the molar concentration of this solution is 100mmol / L;
[0039] (3) get mixed solution after each 10 μ L of the ethanol / water solution of the sucrose solution prepared in step (1) and the ethanol / water solution of step (2) naphthalene ethylenediamine nitrate, and obtain mixed solution, the mol ratio of naphthalene ethylenediamine nitrate and sucrose in the mixed solution The ratio is 10:1; then draw 1 μL of the above mixed solution to apply the sample, and inject the sample after the solvent in the mixed so...
Embodiment 3
[0042] Embodiment 3, with the naphthalene ethylenediamine nitrate prepared in embodiment 1 as the molecular weight of substrate measurement Gly-Ala
[0043] (1) Dissolving 2.48 mg of Gly-Ala with 1.697 ml of ethanol / water solution with a volume ratio of 30:70 to obtain a molar concentration of Gly-Ala solution of 10 mmol / L;
[0044] (2) directly use the ethanol / water solution of the naphthalene ethylenediamine nitrate prepared by embodiment 1, the molar concentration of this solution is 100mmol / L;
[0045] (3) get the solution of Gly-Ala prepared by step (1) and the ethanol / water solution of step (2) naphthalene ethylenediamine nitrate and mix each 10 μ L to obtain the mixed solution, and in this mixed solution, naphthalene ethylenediamine nitrate and Gly- The molar ratio of Ala is 10:1; then draw 1 μL of the above mixed solution to apply the sample, and inject the sample after the solvent in the mixed solution is completely evaporated in the air. It was analyzed by MALDI-TOF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com