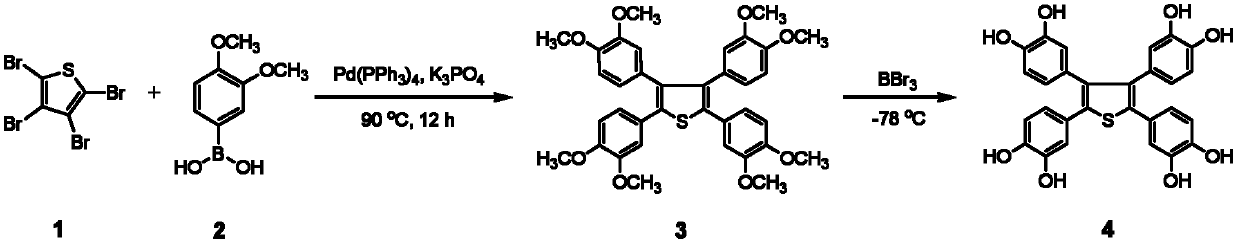
2,3,4,5,-tetra(3',4'-dihydroxyl phenyl)thiophene and application thereof as MALDI (matrix assisted laser desorption ionization) matrix in analyzing small molecules
A matrix and matrix-assisted laser technology, which is applied in the analysis of materials, material analysis by electromagnetic means, and measurement devices, can solve the problems of cumbersome preparation, long time consumption, and high cost, and achieve good sample compatibility and reduce requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, synthesis 2,3,4,5-tetra(3 ', 4'-dihydroxyphenyl) thiophene
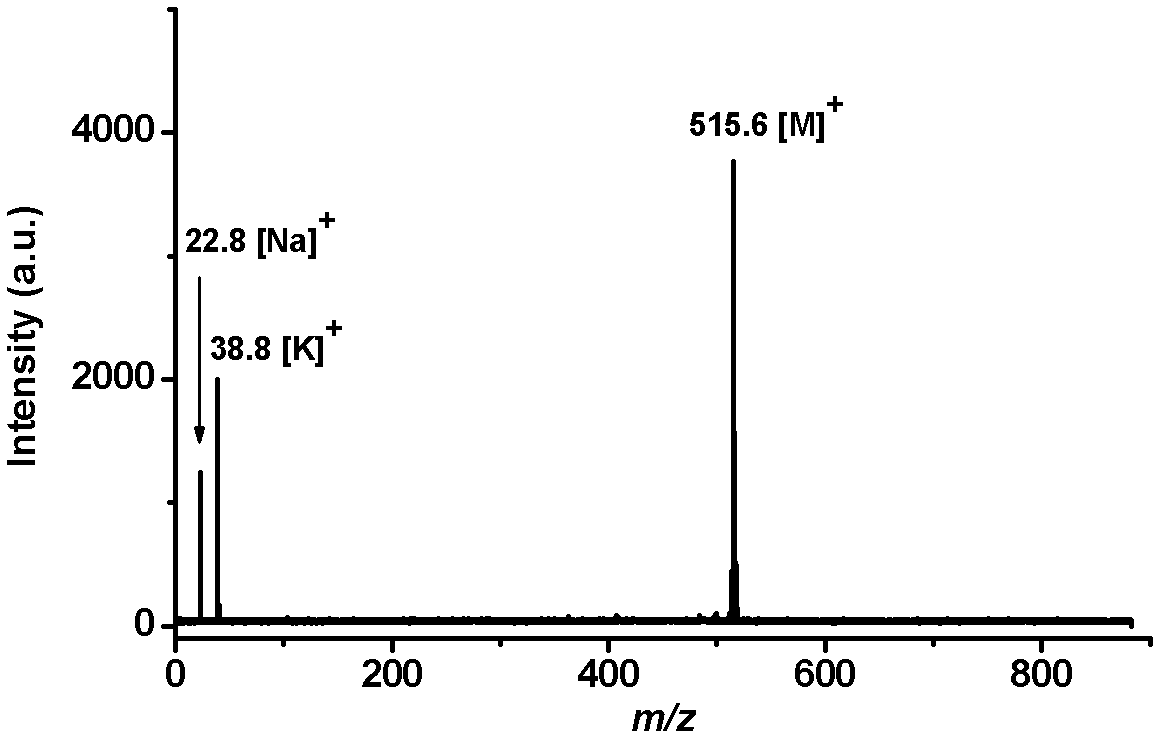
[0054] Add Pd(PPh 3 ) 4 (0.07g, 6mol%), after stirring at room temperature for 30 minutes, add 3,4-dimethoxyphenylboronic acid (2,5.0mmol), K 3 PO 4 (4.0 mmol) and 1 mL of water. The mixture was stirred at 90°C for 12 hours, and after cooling to room temperature, it was successively diluted with ethyl acetate, dried over anhydrous sodium sulfate, and then filtered. After the solution was removed from the solvent by a rotary evaporator, the residue was separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:50, v / v) to obtain the intermediate product 2,3,4,5-tetra(3', 4'-Dimethoxyphenyl)thiophene solid (3, 0.56g, 0.9mmol), yield 90%. 3 (0.56 g, 0.9 mmol) was dissolved in 5 mL of dichloromethane, then cooled to -78 °C. Under these conditions, 2.5 mL of a dichloromethane solution of boron tribromide (0.68 mL, 7.2 mmol) was added dropwise, stirred for 2 hours, returned to...
Embodiment 2
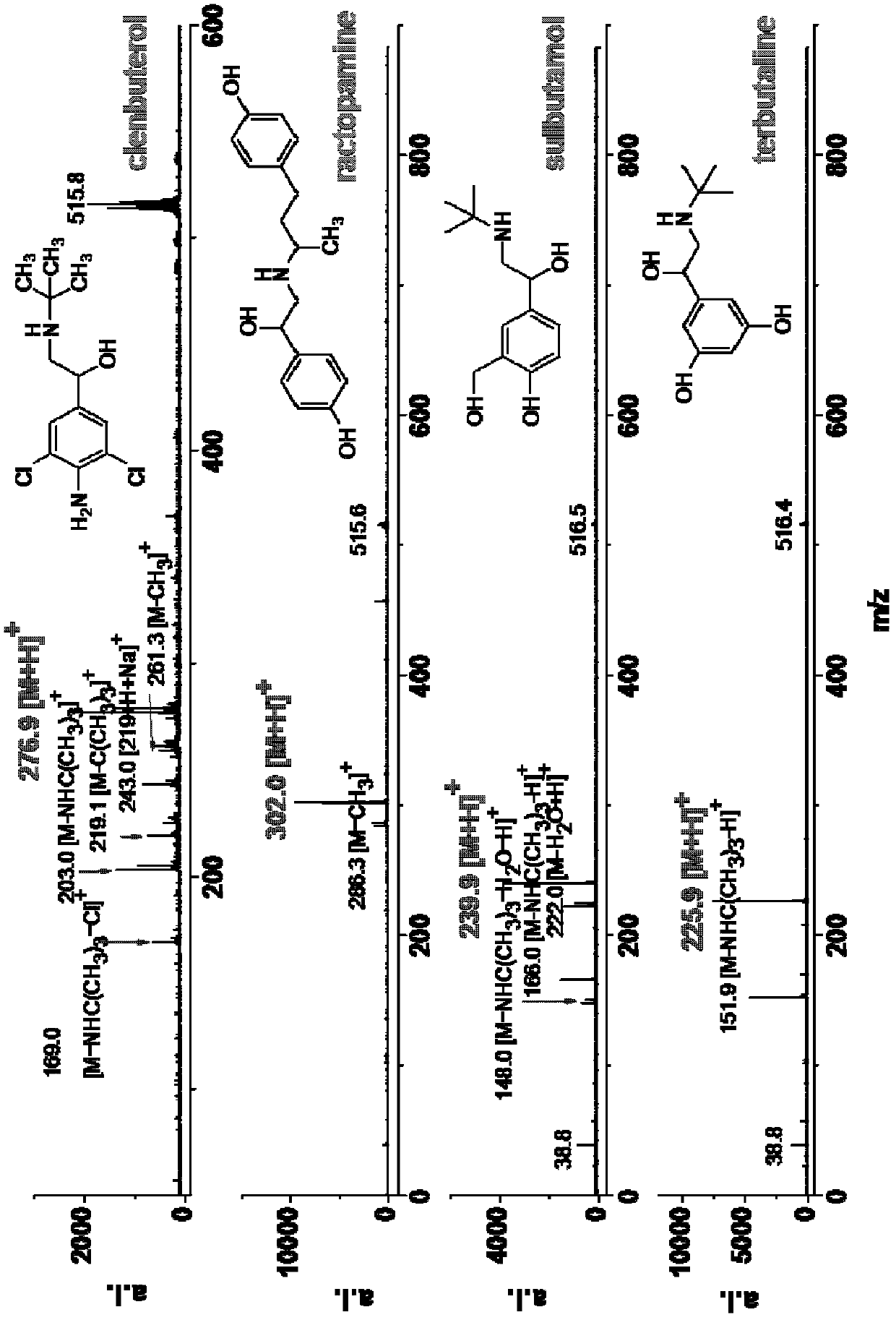
[0057] Example 2. Analysis of various small molecules (including β-stimulants, amino acids, vitamins, caffeine, urea, dopamine, melamine, anilines, purines, pyrimidines and other amines)
[0058] Various prepared sample solutions (concentration: 1mM) and matrix solution (concentration: 20mM) were mixed uniformly at a volume ratio of 1:1, and then 1 μL of the mixed sample was added to the MALDI target plate, dried in the air and then subjected to mass spectrometry analysis. The mass spectrometry conditions are: voltage: accelerating voltage: 19.000kv; delayed extraction voltage: 14.920kv; reflector voltage: 20.000kv; lens voltage: 7.000kv; frequency: 1.000Hz; laser energy: 75-80%; accumulation times: 90 times ; Positive ion mode.
[0059] In the MALDI ionization mode, the matrix can transfer protons to the analyte compound, making the analyte compound positively charged and thus detected by mass spectrometry. Experiments have shown that the matrix is effective for the above ...
Embodiment 3
[0060] Embodiment 3, small molecule analysis in urine sample
[0061] Take 2 mL of fresh urine from healthy male volunteers, centrifuge to remove precipitates and insoluble matter, then take 1 μL and mix it with 1 μL matrix solution (concentration: 20 mM), and finally take 1 μL of the mixed solution and add it to the MALDI target plate, dry it in the air and enter the mass spectrometer analyze. The mass spectrometry conditions are: voltage: accelerating voltage: 19.000kv; delayed extraction voltage: 14.920kv; reflector voltage: 20.000kv; lens voltage: 7.000kv; frequency: 1.000Hz; laser energy: 75-80%; accumulation times: 90 times ; Positive ion mode.
[0062] From Figure 8 It can be seen from the figure that more signal peaks are generated in the small molecule region, indicating that many small molecules in the urine sample are ionized to generate mass spectrometry signals. Therefore, this matrix can be used for the analysis of small molecule metabolites in urine samples....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com