Vaginal drug administration device comprising clotrimazole, metronidazole and chlorhexidine and preparing method thereof
A technology for vaginal administration and clotrimazole is applied in the field of preparation of the drug administration device, and can solve the problems of gastrointestinal reaction, leukopenia, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
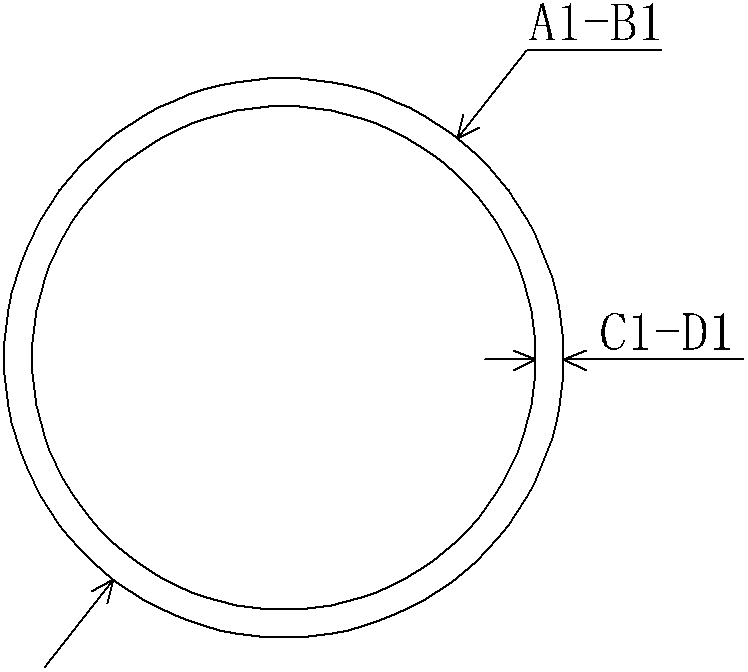
[0039] Use an injection molding machine to inject medical polyethylene (molecular weight 5000-21000) into an "O"-shaped bracket with an outer diameter of 40mm and a thickness of 2mm, such as figure 1 shown.
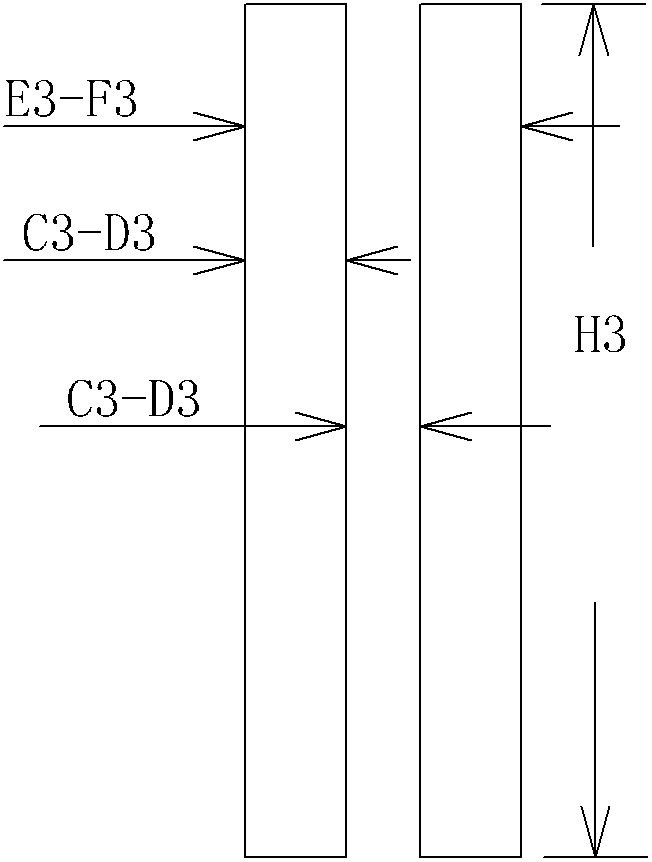
[0040] Such as figure 2 As shown, prepare an pearl cotton drug-loaded tube (purchased from Shanghai Si Ding Buffer Material Co., Ltd.) with an outer diameter of 9 mm, a thickness of 3.5 mm, and a length of 140 mm, and put the drug-loaded tube on the obtained "O" type stent .
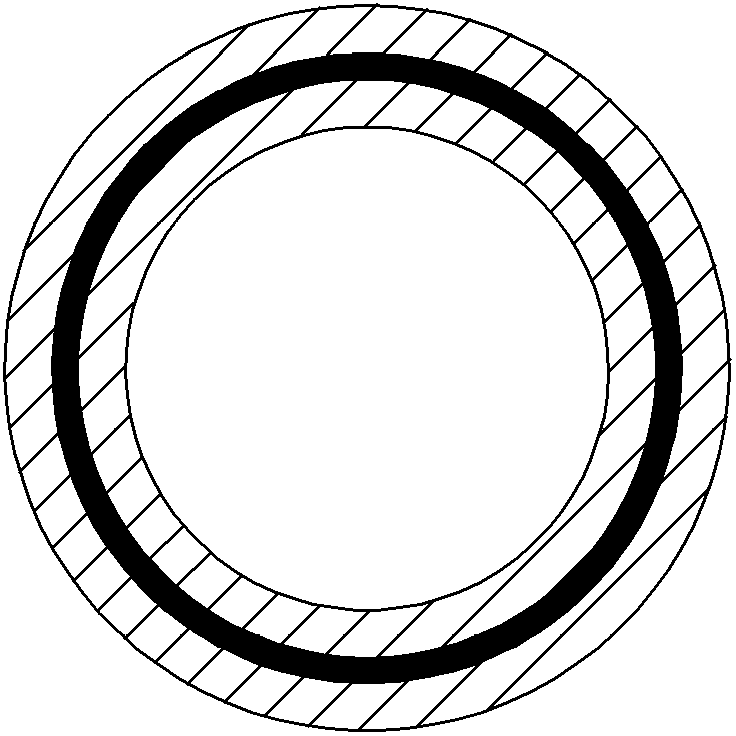
[0041] Weigh 1g of metronidazole, 50mg of chlorhexidine, 0.3g of ethyl cellulose, and 4g of ethanol, mix the four evenly, and impregnate them on the "O"-shaped support of the polyurethane sponge drug-loaded tube prepared above to obtain A containing drug layer.
[0042] Weigh 0.3 g of clotrimazole, 0.2 g of sodium carboxymethyl cellulose, and 3 g of ethanol, mix the three evenly, and impregnate them on the outside of the drug-containing layer of A to obtain the drug-containing layer of B.
[00...
Embodiment 2
[0045] Use an injection molding machine to inject medical polyethylene material (molecular weight 2000-21000) into an "O" type stent. Its outer diameter is 4.5mm and its thickness is 2mm.
[0046] Such as figure 2 As shown, prepare a polyurethane sponge drug-loading tube with an outer diameter of 8 mm, a thickness of 4 mm, and a length of 140 mm (purchased from Shanghai Siding Buffer Material Co., Ltd.), and put the drug-loading tube on the obtained "O"-shaped stent.
[0047] Weigh 1g of metronidazole, 50mg of chlorhexidine, 0.1g of cellulose acetate phthalate, and 4g of ethanol, mix the four evenly, and impregnate them on the "O"-shaped support of the polyurethane sponge drug-loaded tube prepared above, A drug-containing layer is obtained.
[0048] Weigh 0.5 g of clotrimazole, 0.3 g of hydroxypropyl methylcellulose, and 4 g of ethanol, mix the three evenly, and impregnate them on the outside of the drug-containing layer of A to obtain the drug-containing layer of B.
[00...
Embodiment 3
[0051] The polyurethane material is injected into an "O"-shaped bracket with an injection molding machine, with an outer diameter of 4.0mm and a thickness of 2mm.
[0052] Such as figure 2 As shown, prepare a polyurethane sponge drug-loaded tube (purchased from Shanghai Si Ding Buffer Material Co., Ltd.) with an outer diameter of 7 mm, a thickness of 2.8 mm, and a length of 12.3 mm, and put the drug-loaded tube on the obtained "O" type stent superior.
[0053] Weigh 1.2g of metronidazole, 45mg of chlorhexidine, 0.1g of acrylic resin, and 5g of ethyl acetate, mix the four evenly, and impregnate them on the "O"-shaped support of the polyurethane sponge drug-loaded tube prepared above to obtain A Drug-containing layer.
[0054] Weigh 0.4 g of clotrimazole, 0.2 g of hydroxypropyl methylcellulose, and 5 g of water, mix the three evenly, and impregnate them on the outside of the drug-containing layer of A to obtain the drug-containing layer of B.
[0055] Weigh 0.3 g of methyl v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com