Antigen epitope for preventing and treating trichinosis, composition thereof and application thereof
A technology of antigenic epitope and composition, applied in the field of immunobiology, can solve the problem of in-depth research on Trichinella paramyosin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of rTs-Pmy-N1-322aa protein
[0056] As shown in the sequence of steps 1-6 below.
[0057] 1. Amplification of the Ts-Pmy-N1-322aa gene fragment
[0058]According to the reading frame sequence of paramyosin gene (Ts-Pmy GenBank accession No.: EF429310), the nucleotide sequence of Ts-Pmy-N1-322aa can be obtained as follows:
[0059] ATGTCTCTGTATCGCAGTCCCAGTGCGTCAGTGATGAGATCAGCAAGCATGCTCAGCCGAAGTGGCGGATTCGATGCTTACGGATTTGGAGGTTACGGTGCGCCAAGCCTCAACGTTGCCGACTTGGGTTCTTTGACCAGACTCGAGGATAAAATTCGCCTGCTTCAAGATGATTTGGAAACGGAAAGAGAATTGCGAAACCGAATTGAACGCGAACGTGCCGATTTGTCCTGCCAACTGATCAGCTTAACCGATCGATTGGAAGAGGCTGAAGGAACCACCGATGCCCAGATCGACGCCAATCGAAAGCGTGAATCCGAATTGCAAAAGTTGAGAAAAATATTGGAAGATTCGCAATTGGAAAGCGAAGATTCGCTGAACCAGCTGCGCAAGAAGCACCAAGAATCCCTTTTAGATTATCAGCAGCAAATTGAACAACTTCAAAAGAAAAATAGCAAAATCGACAGAGAACGACAACGTTTGCAGCATGAAGTCATTGAACTTACTGCCGGAATTGATCAGATGCAAAAAGACAAGCATGCCGCGGAAAAAGCTGCCGAAAAGCACGAAGCGCATGCCAGAGAGCTTCAGAACAGAGTTGACGATCTGGCAAAAAATTTGA...
Embodiment 2
[0118] Embodiment 2: the establishment of hybridoma cell line
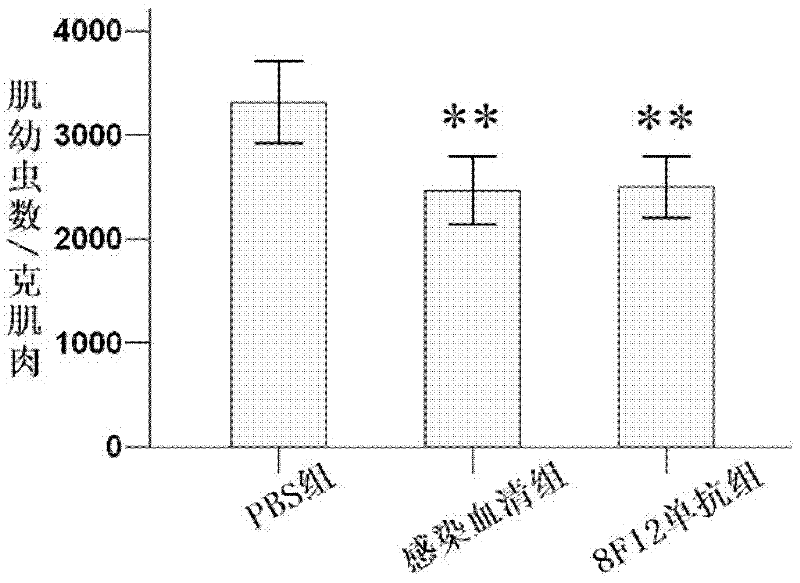
[0119] The rTs-Pmy-N1-322aa protein (solution) prepared in Example 1 was used to immunize BALB / c mice. After 4 times of immunization, the mouse antibody titer reached 1:128000 after the last immunization. Splenocytes from immunized mice were fused with SP2 / 0 myeloma cells in logarithmic growth phase, and cultured in HAT medium. In the 96-well culture plate, the growth of fusion cells can be seen. When the cell colony grows to 1 / 3 well, it is identified by indirect ELISA and Western blot, and the anti-rTs-Pmy-N1-322aa antibody is selected in the culture supernatant. It is also a positive hybridoma cell line that can recognize rTs-Pmy-N1-322aa and larva protein. After 3 times of subcloning, a hybridoma cell line was screened and named 8F12. The antibody secreted by it belonged to IgG1 subclass antibody secreting Th2 type. After continuous subculture for 20 generations, F5, F10, F15 and F20 were selected respectiv...
Embodiment 3
[0123] Embodiment 3: Identification of the monoclonal antibody (mAb) secreted by 8F12 cell line
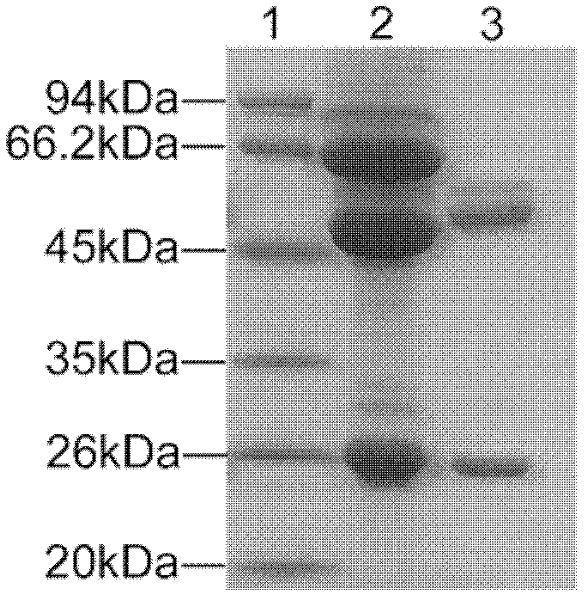
[0124] The titers of mAb in the cell culture supernatant and ascitic fluid of the 8F12 cell line in Example 2 detected by ELISA were 1:3200 and 1:64000, respectively. The secreted antibody subclasses were all IgG1 subclass κ type (Table 3). The monoclonal antibody ascites was purified by HiTrap rProtein A column and then subjected to SDS-PAGE electrophoresis. Bands appeared at 50kDa and 25kDa respectively, which corresponded to the position of IgG antibody heavy chain and light chain ( figure 1 ).
[0125] Table 3: Identification of monoclonal antibody 8F12
[0126]
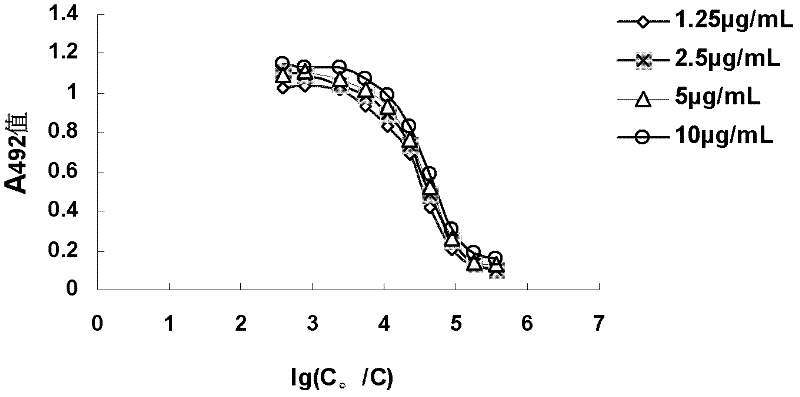
[0127] In order to clarify the relative affinity of the mAb when it binds to the antigen at a certain antigen concentration, an indirect non-competition ELISA method was used. The logarithmic value of the dilution factor is 1g (C 0 / C) is the abscissa (C 0 is the initial concentration of mAb, and C is the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com