Non-aqueous electrolyte and lithium secondary battery including the same
A lithium secondary battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as severe heat, fire and burning, and a large amount of gas ejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] First, 3% oligomer of phenylmethane maleimide (compound (A)) was dissolved in EC / PC to form a mixed solution. Next, 2,4-dimethyl-2-imidazoline (2,4-bimethyl-2-imidazoline) (compound (B)) was added in batches to the mixed solution, and the heating polymerization reaction was carried out at 130° C. for 8 hours, wherein The molar ratio of 3% benzyl maleimide oligomer to 2,4-dimethyl-2-imidazoline is 2:1. So far, the metastable nitrogen-containing polymer of Example 1 was obtained.
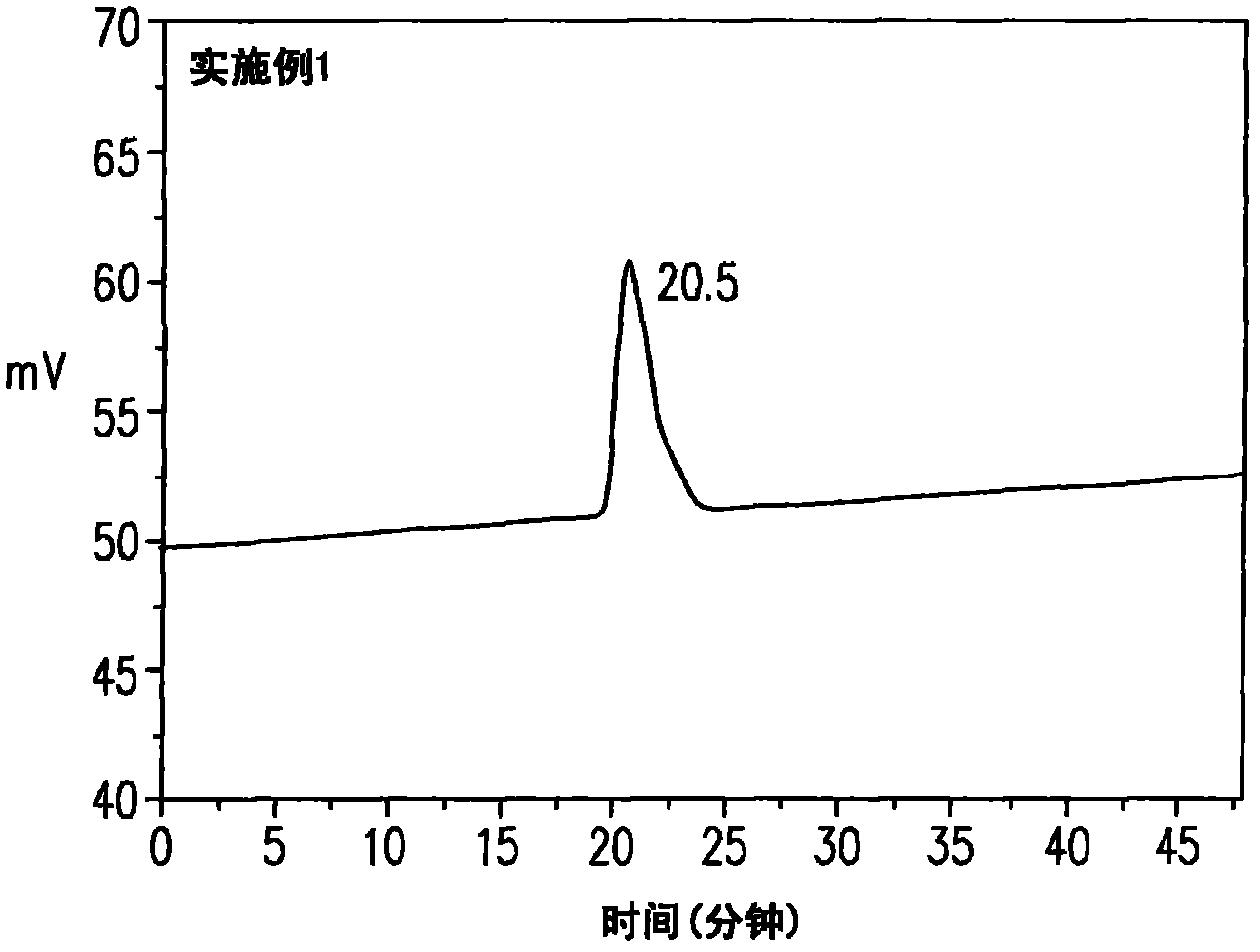
[0084] The metastable nitrogen-containing polymer of embodiment 1 is a polymer with a narrow molecular weight distribution, its GPC gel permeation chromatography peak time is 20.5 minutes, and the molecular weight distribution index (Polydispersity index, PDI) 1.2, such as figure 1 shown. In addition, the metastable nitrogen-containing polymer in Example 1 was induced again at a temperature of 186° C., so that the metastable nitrogen-containing polymer was completely converted into a macromo...
Embodiment 2
[0086] First, 5% 4,4'-diphenylmethane bismaleimide (compound (A)) was dissolved in GBL to form a mixed solution. Next, 2,4-dimethyl-2-imidazoline (compound (B)) was added in batches to the mixed solution, and thermal polymerization was carried out at 100°C for 15 hours, wherein 5% of 4,4'-diphenylmethane bis The molar ratio of maleimide to 2,4-dimethyl-2-imidazoline is 2:1. So far, the metastable nitrogen-containing polymer of Example 2 was obtained.
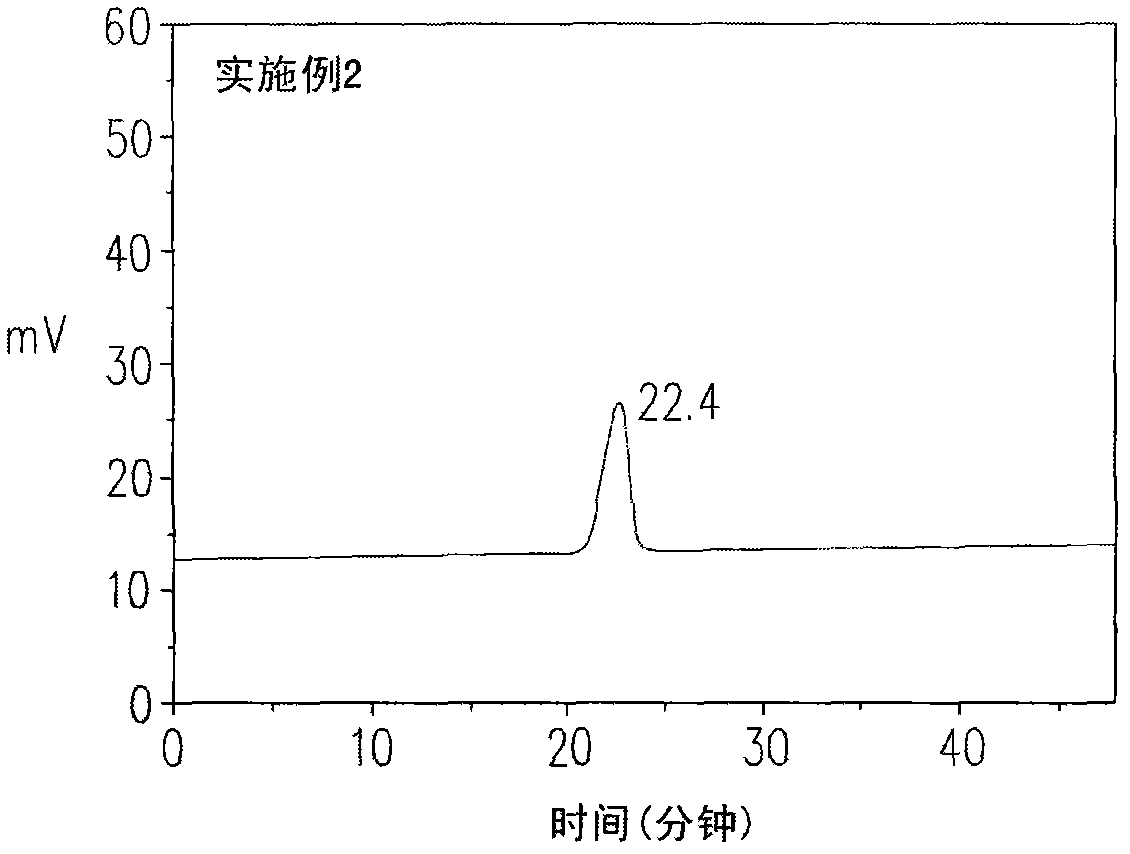
[0087] The metastable nitrogen-containing polymer of embodiment 2 is a kind of polymer of narrow molecular weight distribution, and its GPC peak time is 22.4 minutes, molecular weight distribution index (PDI) 1.2, as figure 2 shown. In addition, the metastable nitrogen-containing polymer of Example 2 was induced again at a temperature of 180° C., so that the metastable nitrogen-containing polymer was completely converted into a macromolecular polymer.
Embodiment 3
[0089] First, 3% oligomer of benzyl maleimide (compound (A)) was dissolved in NMP to form a mixed solution. Next, 2,4-dimethyl-2-imidazoline (compound (B)) was added in batches to the mixed solution, and thermal polymerization was carried out at 150°C for 3 hours, in which 3% of benzenemethane maleimide oligo The molar ratio of polymer to 2,4-dimethyl-2-imidazoline was 4:1. So far, the metastable nitrogen-containing polymer of Example 3 was obtained.
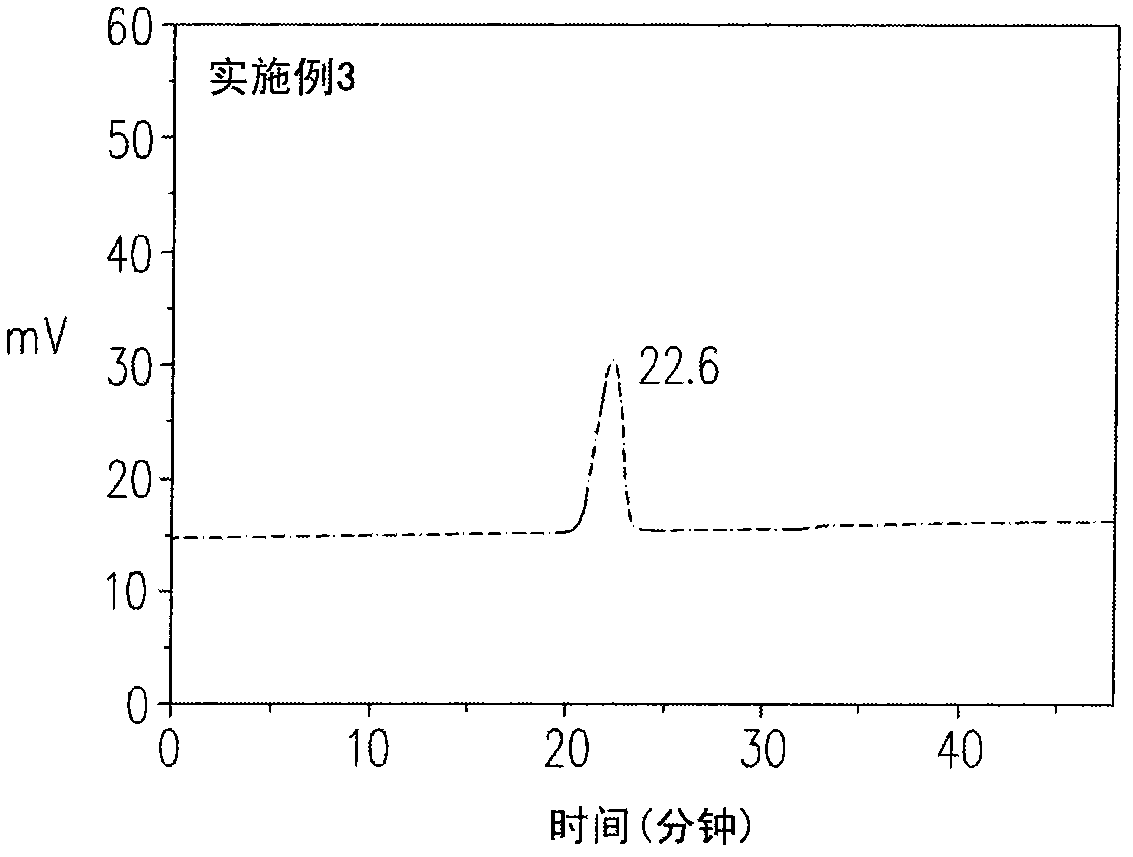
[0090] The metastable nitrogen-containing polymer of embodiment 3 is a kind of polymer of narrow molecular weight distribution, and its GPC peak time is 22.6 minutes, molecular weight distribution index (PDI) 1.2, as image 3 shown. In addition, the metastable nitrogen-containing polymer in Example 3 was induced again at a temperature of 186° C., so that the metastable nitrogen-containing polymer was completely converted into a macromolecular polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com