Slow release composition with cefaclor
A slow-release composition, cefaclor technology, applied in drug delivery, pill delivery, pharmaceutical formulations, etc., can solve the problems of low blood drug concentration, difficulty in ensuring curative effect, and difficulty in ensuring blood drug concentration in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment is only used for comparison and description, and is not the content of the present invention.
[0035]
[0036] Mix cefaclor with mannitol, hypromellose K4M, and hypromellose K100M evenly, add 5% hypromellose in 80% ethanol aqueous solution to granulate, dry, arrange, and add magnesium stearate Mix well and compress into tablets.
[0037] In this example, only hypromellose is used as the skeleton material, mannitol is used as the porogen, and no pH regulator is used in this prescription.
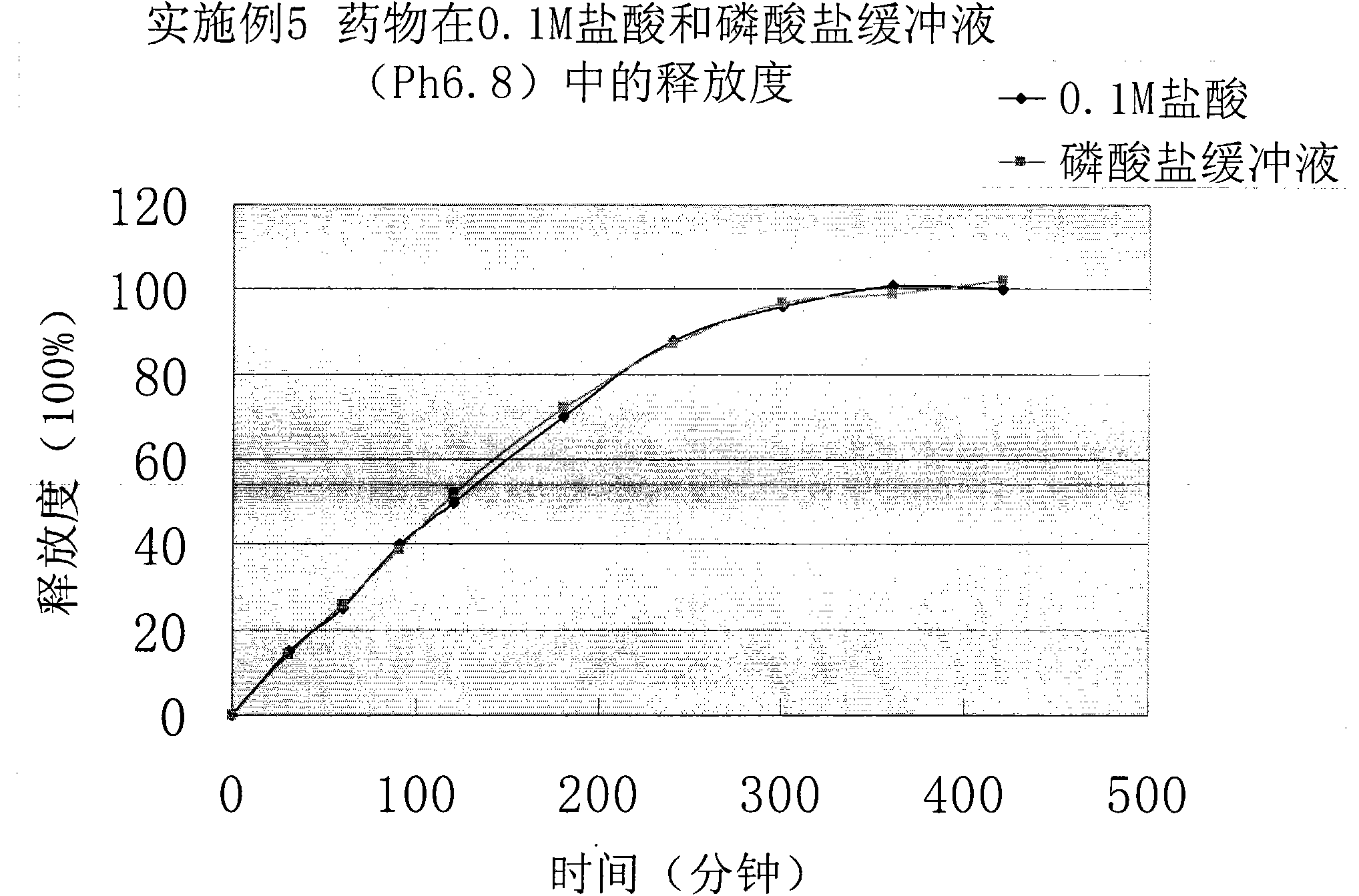
[0038] Get this product, according to the release assay method (Chinese Pharmacopoeia 2010 edition two appendix X D first method), adopt the device of dissolution assay method first method, respectively with 0.1mol / L hydrochloric acid solution (instead of artificial gastric juice, the same below) , Phosphate buffer (pH6.8) (replacing artificial intestinal juice, the same below) as the release medium, the speed is 100 rpm, operated according to the law, at 30min, 60...
Embodiment 2
[0041] This embodiment is only used for comparison and description, and is not the content of the present invention.
[0042]
[0043] Mix cefaclor with lactose, hypromellose K100M and acrylic resin (Eudragi tL-100-55), add 6% povidone K30 in 70% ethanol aqueous solution to granulate, dry, arrange, add hard Magnesium fatty acid is mixed and pressed into tablets.
[0044] In this case, hypromellose is used as the framework material, lactose as the porogen, and acrylic resin (Eudragit L-100-55) as the pH regulator.
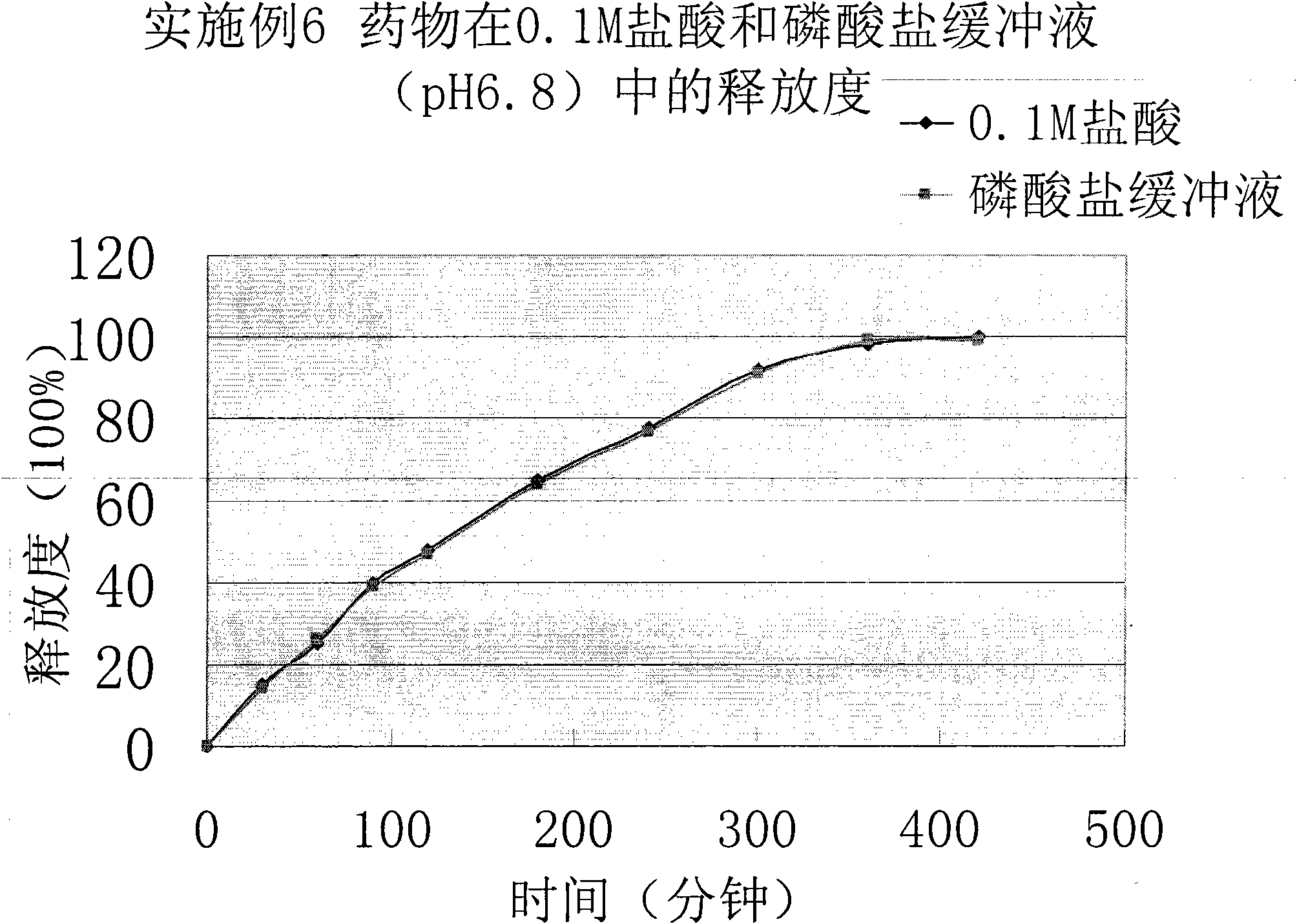
[0045] Get this product, according to the release assay method (Chinese Pharmacopoeia 2010 edition two appendix X D the first method), adopt the device of the dissolution assay method first method, respectively with 0.1mol / L hydrochloric acid solution, phosphate buffer saline (pH6. 8) For the release medium, the rotating speed is 100 revolutions per minute, operated according to the law, sampling at 30min, 60min, 90min, 120min, 180min, 240min, 300min, 360min, 42...
Embodiment 3
[0048] This embodiment is only used for comparison and description, and is not the content of the present invention.
[0049]
[0050] After mixing cefaclor with lactose, hypromellose K100M, and acrylic resin (Eudragit L-100-55), add 6% povidone K30 in 70% ethanol aqueous solution to granulate, dry, arrange, and add stearin Magnesium acid mixed, pressed into tablets.
[0051] In this case, hypromellose is used as the framework material, lactose as the porogen, and acrylic resin (Eudragit L-100-55) as the pH regulator.
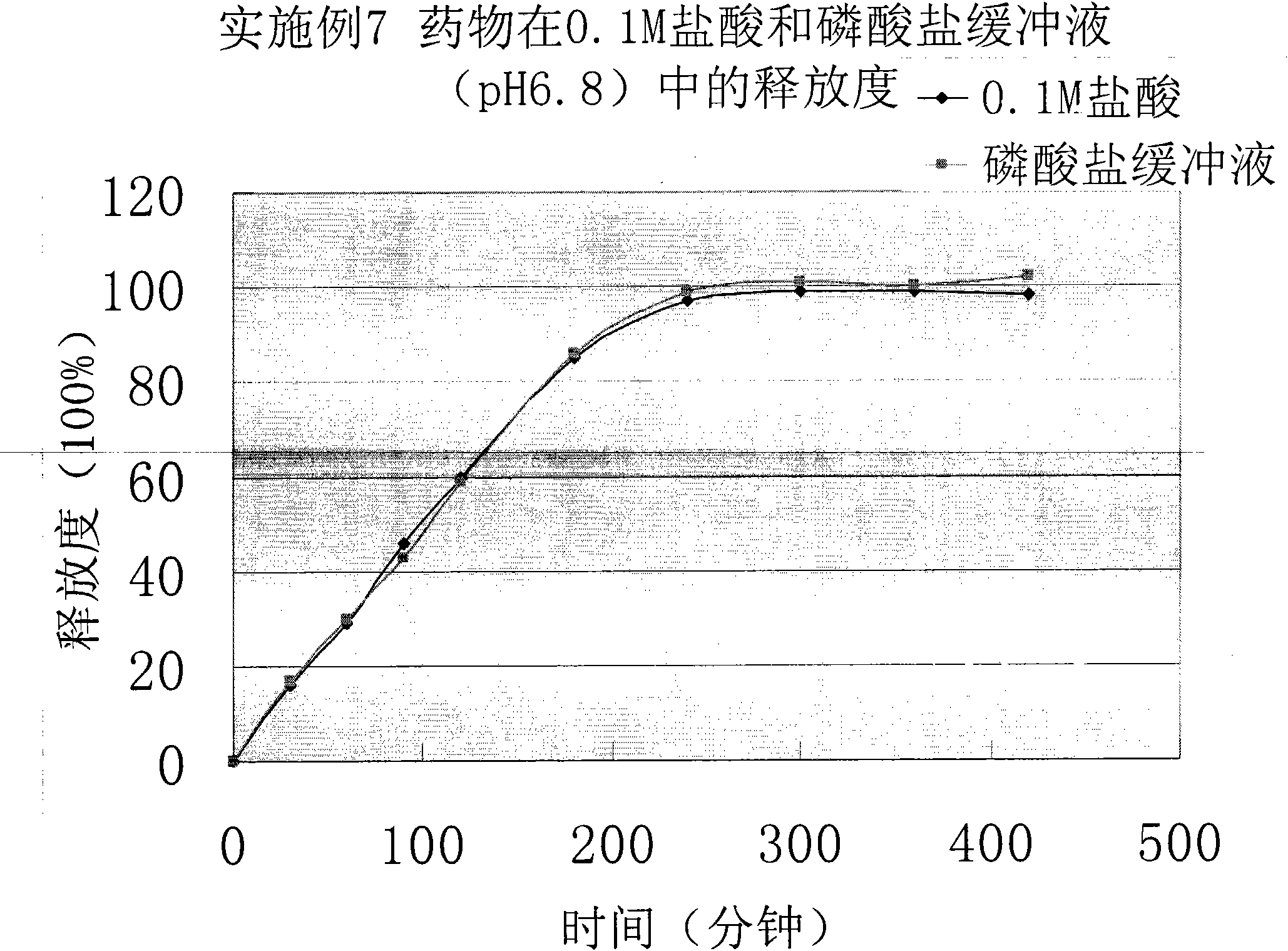
[0052] Get this product, according to the release assay method (Chinese Pharmacopoeia 2010 edition two appendix X D the first method), adopt the device of the dissolution assay method first method, respectively with 0.1mol / L hydrochloric acid solution, phosphate buffer saline (pH6. 8) For the release medium, the rotating speed is 100 revolutions per minute, operated according to the law, sampling at 30min, 60min, 90min, 120min, 180min, 240min, 300min, 360mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com