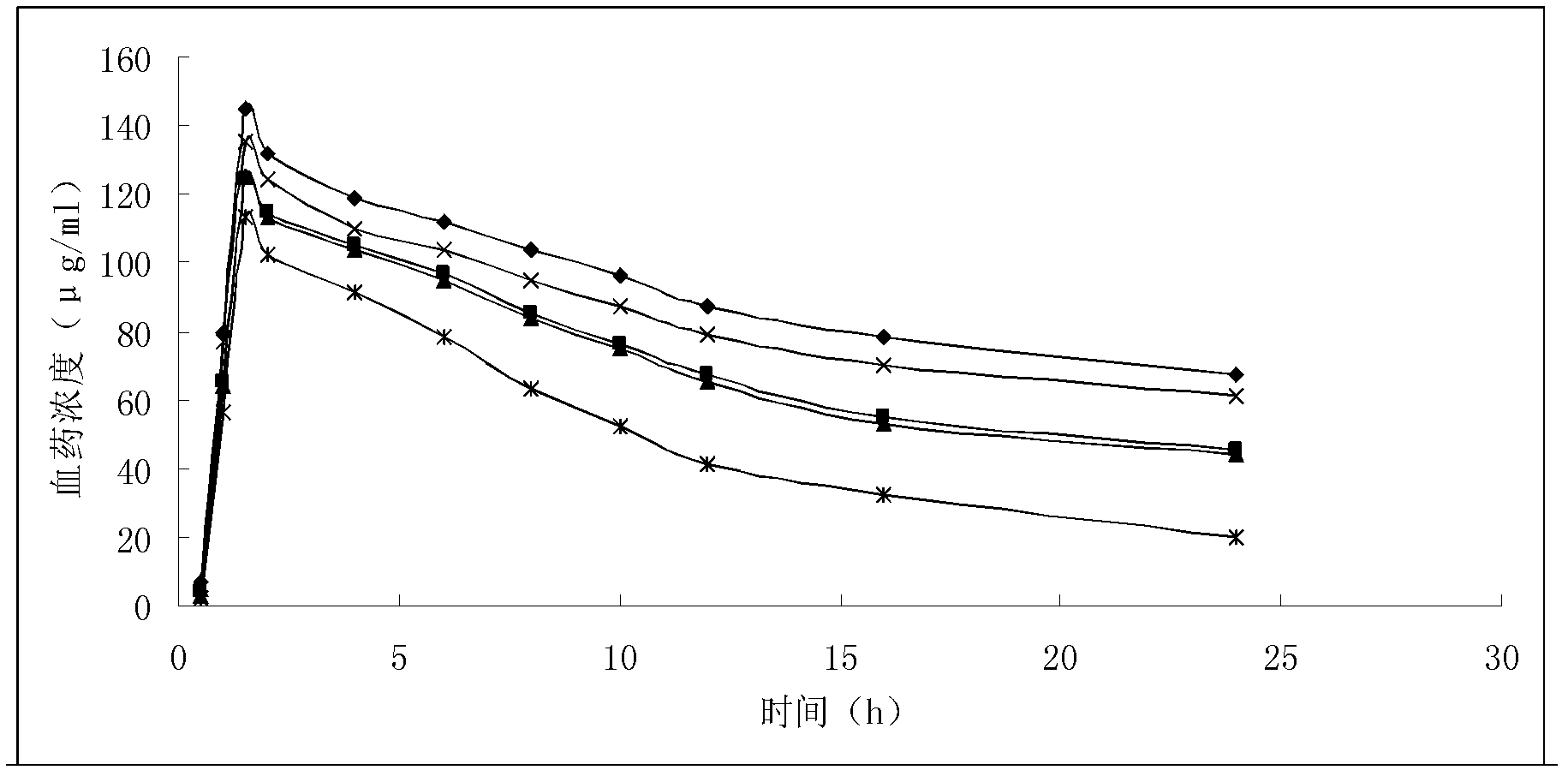
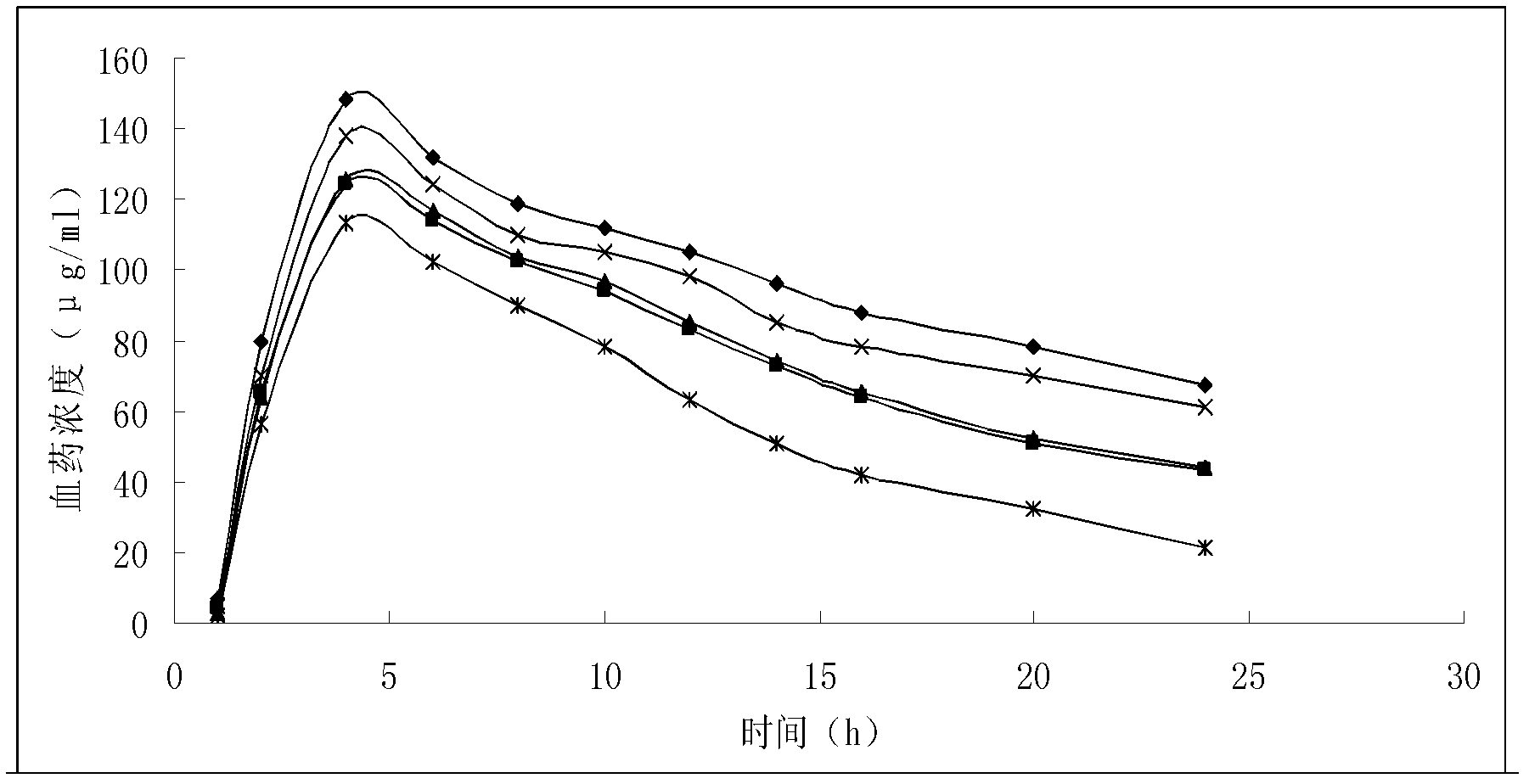
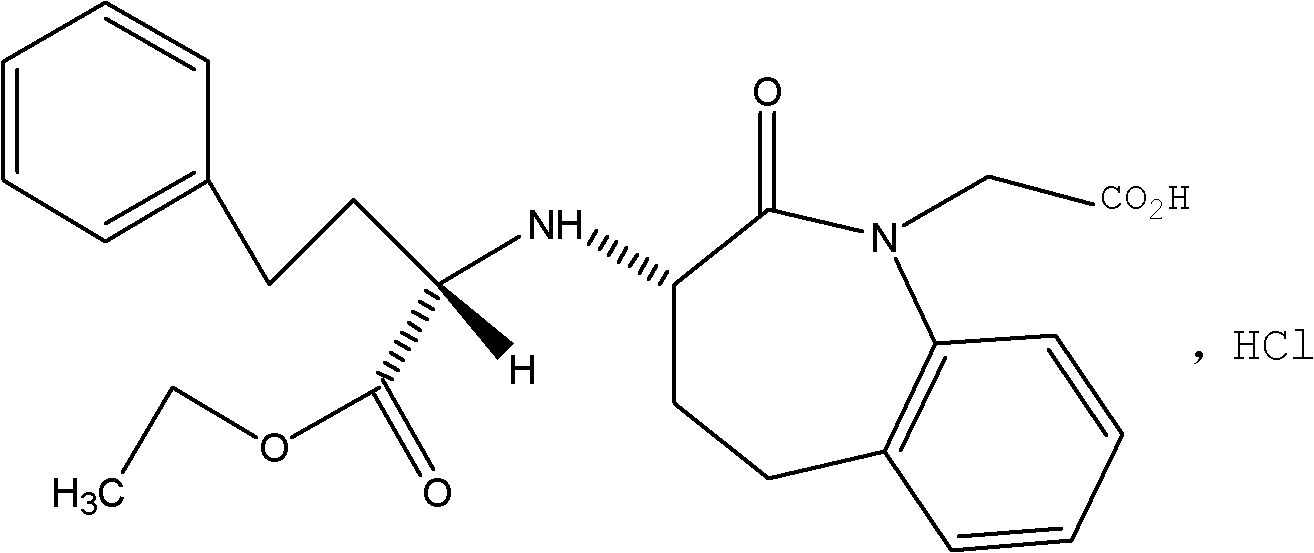
Liposome solid preparation of benazepril/hydrochlorothiazide medicine combination
A technology of liposome preparation and hydrochlorothiazide, which is applied in the field of medicine, can solve the problems of liposome stability and poor encapsulation efficiency, and achieve the effects of excellent dissolution, high stability and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Preparation of Benazepril / Hydrochlorothiazide Liposomal Tablets
[0085] The raw and auxiliary materials used in the prescription (1000 tablets) are as follows:
[0086]
[0087] Preparation Process
[0088] (1) 250g soybean lecithin, 100g octadecylamine and 25g cholesterol acetyl ester are dissolved in the mixed solvent of acetonitrile and dichloromethane that the volume ratio of 1000ml is 2: 3, obtain lipid solution;
[0089] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 55° C. to form a uniform lipid film;
[0090] (3) Disperse 5 g of benazepril hydrochloride and 6.25 g of hydrochlorothiazide in 500 ml of water, add them to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0091] (4) Place the above-mentioned suspension in an ultr...
Embodiment 2
[0096] Example 2 Preparation of Benazepril / Hydrochlorothiazide Liposomal Tablets
[0097] The raw and auxiliary materials used in the prescription (1000 tablets) are as follows:
[0098]
[0099] Preparation Process
[0100] (1) 700g soybean lecithin, 350g octadecylamine and 200g cholesterol acetyl ester are dissolved in the mixed solvent of acetonitrile and dichloromethane that 2000ml volume ratio is 2: 3, obtain lipid solution;
[0101] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 45° C. to form a uniform lipid film;
[0102] (3) Disperse 5 g of benazepril hydrochloride and 6.25 g of hydrochlorothiazide in 500 ml of water, add them to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0103] (4) Place the above-mentioned suspension in an ultrasonic...
Embodiment 3
[0108] Example 3 Preparation of Benazepril / Hydrochlorothiazide Liposomal Tablets
[0109] The raw and auxiliary materials used in the prescription (1000 tablets) are as follows:
[0110]
[0111] Preparation Process
[0112] (1) 600g soybean lecithin, 300g octadecylamine and 80g cholesterol acetyl ester are dissolved in the mixed solvent of acetonitrile and dichloromethane that 1500ml volume ratio is 2: 3, obtain lipid solution;
[0113] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 50° C. to form a uniform lipid film;
[0114] (3) Disperse 10 g of benazepril hydrochloride and 12.5 g of hydrochlorothiazide in 600 ml of water, add them to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0115] (4) Place the above-mentioned suspension in an ultrasonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com