Application of 4-1H-1,2,3-triazole-beta-lactam derivative
A technology of lactam and derivatives, applied in the field of 4-1H-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
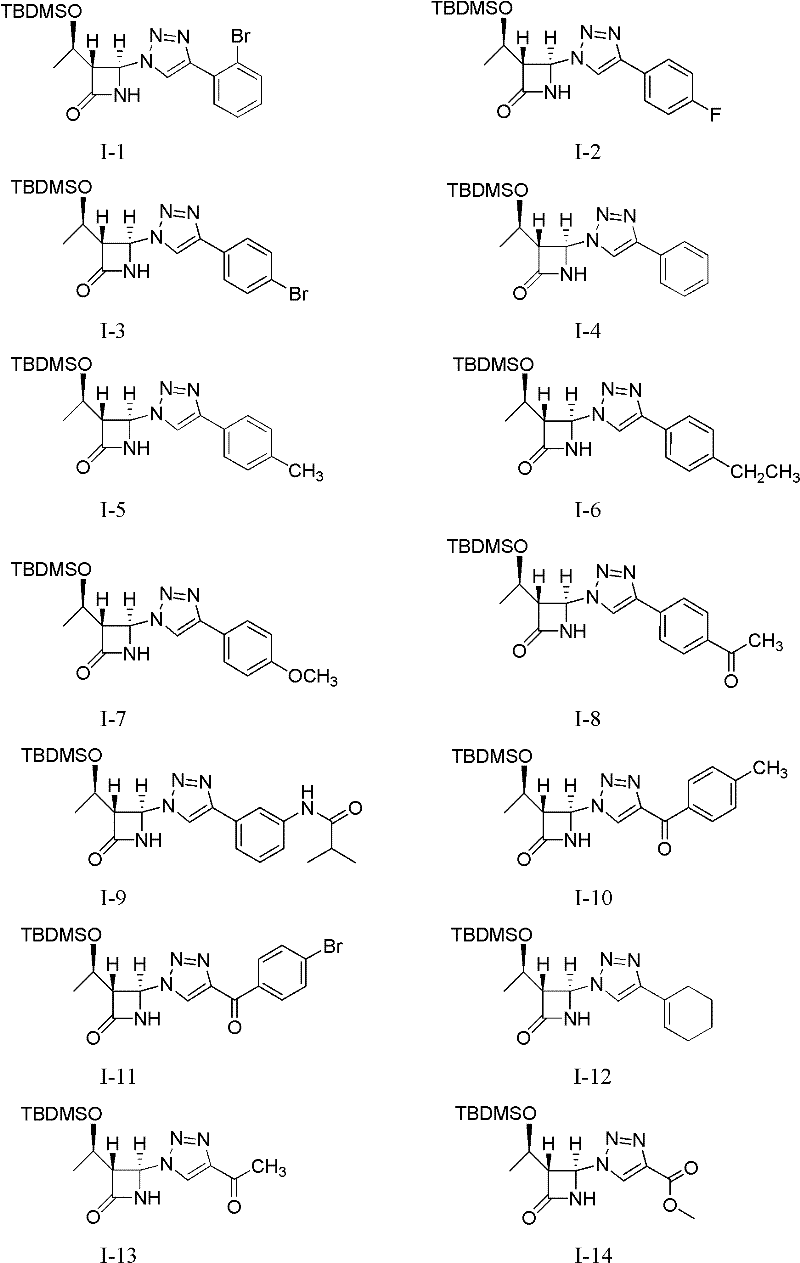
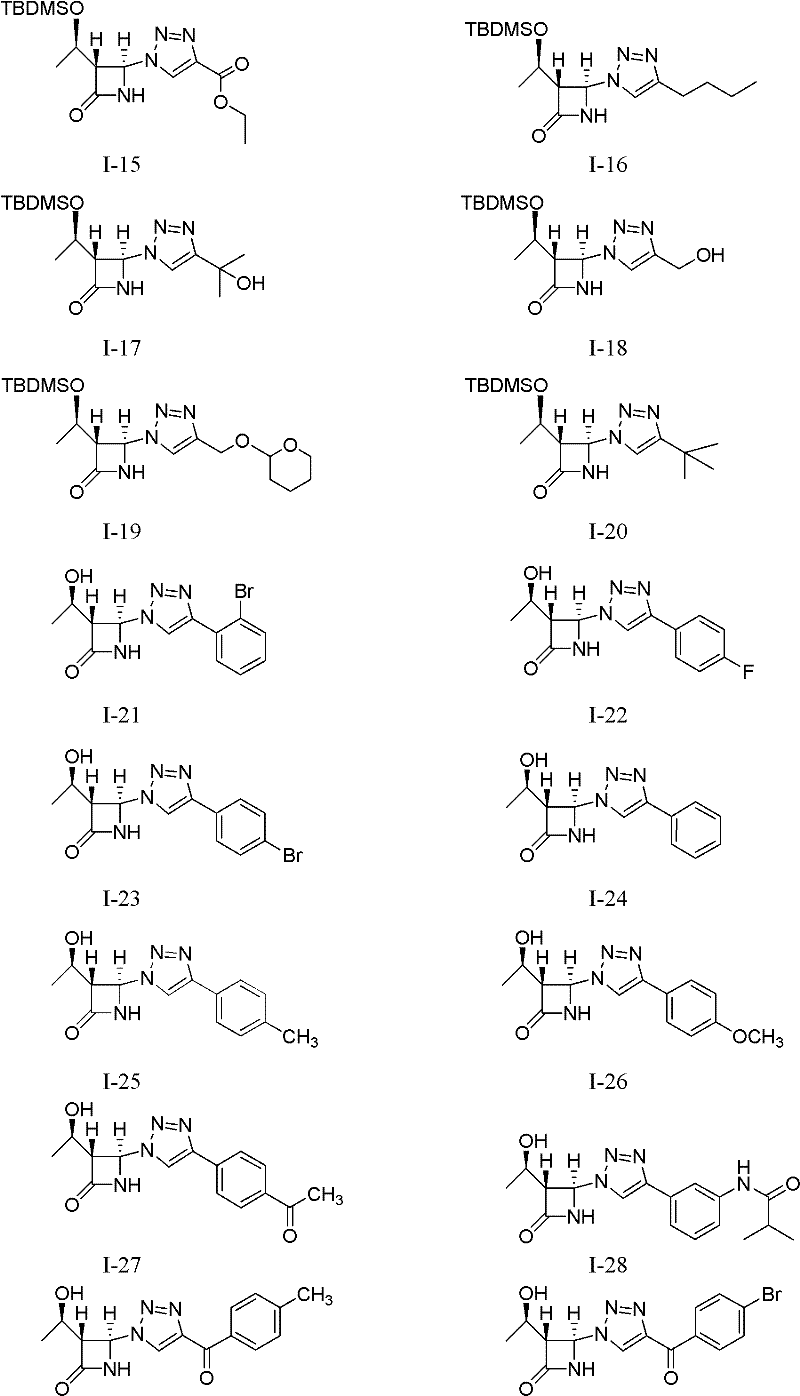
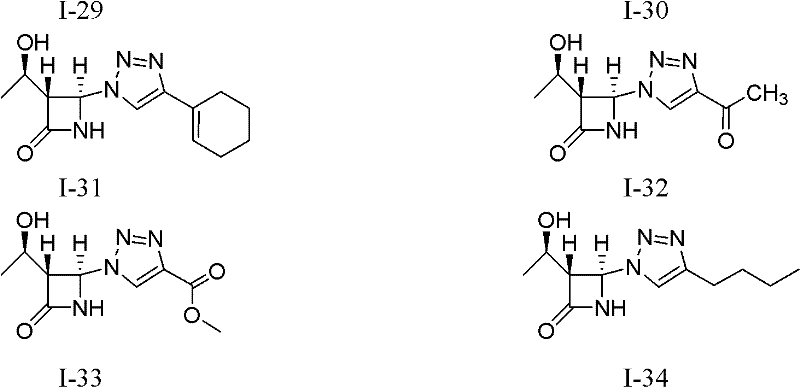
[0023] Example 1 (1'R, 3R, 4R)-4-(4-(2'-bromophenyl)-1 hydrogen-1,2,3-triazol-1-yl)-3-(1'- Preparation of tert-butyldimethylsilyloxy)ethyl-azetidin-2-one (I-1)
[0024]
[0025] This example relates to the general synthesis method of a class of 4-position-1H-1,2,3-triazole-β-lactam derivatives with cytotoxic activity as shown in formula (I), wherein R1 is t-Butyldimethylsilyl (TBDMS). Specifically related to (1'R,3R,4R)-4-(4-(2'-bromophenyl)-1hydro-1,2,3-triazol-1-yl)-3-(1'-tert Preparation of butyldimethylsilyloxy)ethyl-azetidin-2-one (I-1). Add (1'R,3R,4R)-4-acetoxy-3-(1'-tert-butyldimethylsilyloxy)ethyl-azetidin-2-one (0.8610 g, 3mmol), sodium azide (0.2925g, 4.5mmol), CuI (0.0573g, 0.1mmol), o-bromophenylacetylene (0.5973g, 3.3mmol) and 10mL acetonitrile, stirred, heated to 70°C for reaction, reaction After about 12 hours, the heating was stopped, and the reaction solution was cooled to room temperature. The reaction solution was concentrated and filtered, and the ...
Embodiment 2
[0026] Example 2 Preparation of Compound I-2: Using 4-fluorophenylacetylene as a raw material, the reaction operation was the same as in Example 1, and the crude product was purified by column chromatography (petroleum ether: ethyl acetate=2: 1) to obtain a white solid; Mp 147.8 -149.0℃; IR(KBr): 3431, 3208, 2955, 2930, 1770, 1742, 1497, 1232, 839cm -1 ; 1 H NMR (400MHz, CDCl 3 ): δ8.05(1H, s), 7.79-7.75(2H, m), 7.17(1H, br s, -NH), 7.12-7.08(2H, m), 6.32(1H, s, 4-H) , 4.34(1H, m), 3.54(1H, m, 3-H), 1.27(3H, d, J=6.4Hz), 0.89(9H, s), 0.11, 0.10(total 6H, each s); 13 C NMR (100MHz, CDCl 3 ): δ166.5, 164.0, 161.5, 147.7, 127.5, 127.4, 126.1, 126.0, 116.8, 116.0, 115.8, 77.3, 77.0, 76.6, 68.4, 64.0, 63.1, 25.6, 22.1, 17.8, -4.4, -5.2; MS (ESI): 412.9 [M+Na + ], 390.9[M+H + ].
Embodiment 3
[0027] The preparation of embodiment 3 compound I-3: take 4-bromophenylacetylene as raw material, reaction operation is the same as embodiment 1, crude product column chromatography (petroleum ether: ethyl acetate=4: 1) purifies and obtains white solid; Mp 165.5 -166.5℃; IR(KBr): 3211, 2958, 2930, 1773, 1743, 829cm -1 ; 1 H NMR (400MHz, CDCl 3 ): δ8.05(1H, s), 7.74-7.71(2H, m), 7.60-7.57(2H, m), 6.58(1H, br s, -NH), 6.32(1H, d, J=0.8Hz , 4-H), 4.39(1H, m), 3.54(1H, t, J=2.4Hz, 3-H), 1.30(3H, d, J=6.4Hz), 0.92(9H, s), 0.14, 0.13 (total 6H, each s); 13 C NMR (125MHz, CDCl 3 ): δ166.5, 147.9, 132.4, 129.2, 127.5, 122.8, 117.3, 77.5, 77.3, 77.0, 68.9, 64.3, 63.4, 25.9, 22.4, 18.2, -4.0, -4.9; MS (ESI): 452.0 [M +H + ].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com