Preparation method for trans-glycyrrhizic acid
A technology of glycyrrhizic acid and methyl glycyrrhizic acid, which is applied to the preparation of sugar derivatives, chemical instruments and methods, and steroids, can solve the problems of low yield, difficult operation, unsuitable recovery of cis-glycyrrhizic acid, etc., and achieve High yield, less side reactions, solve the effect of equipment post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
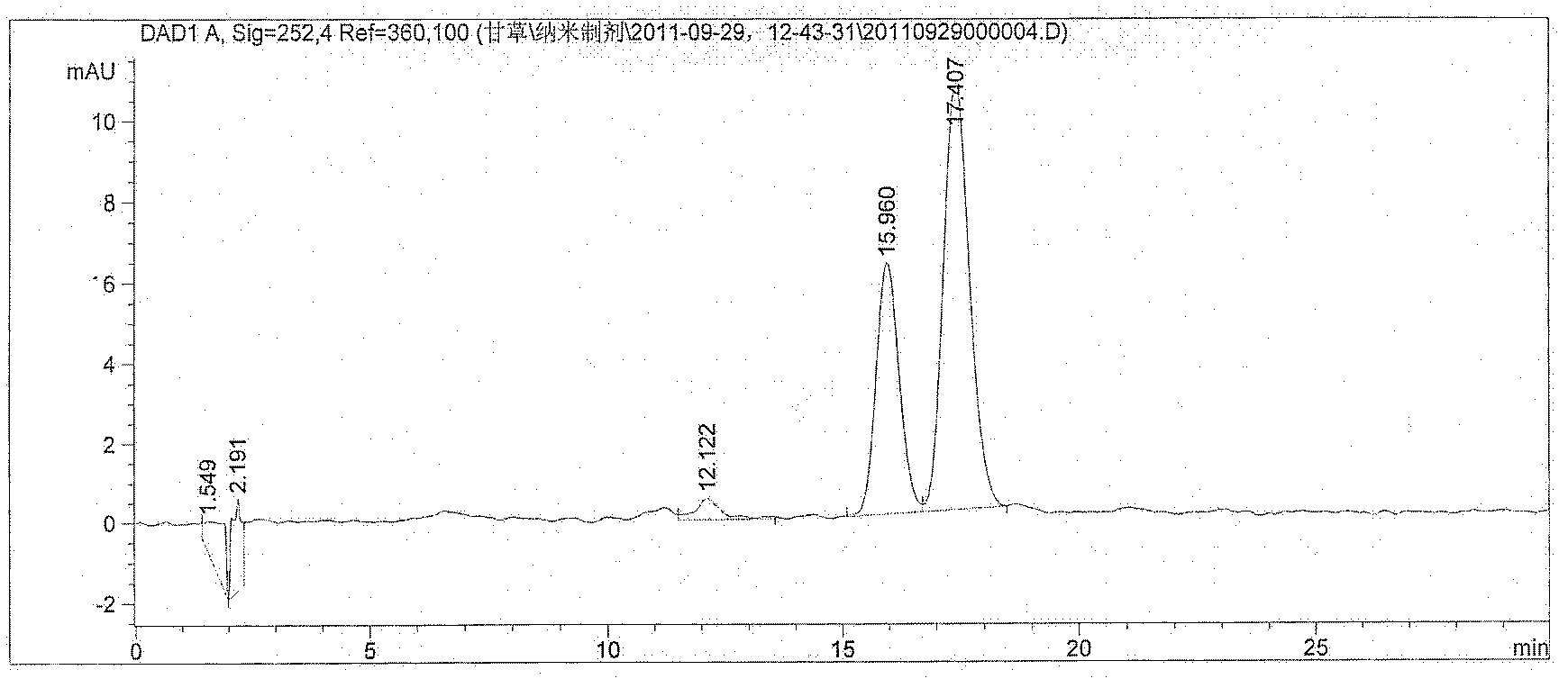
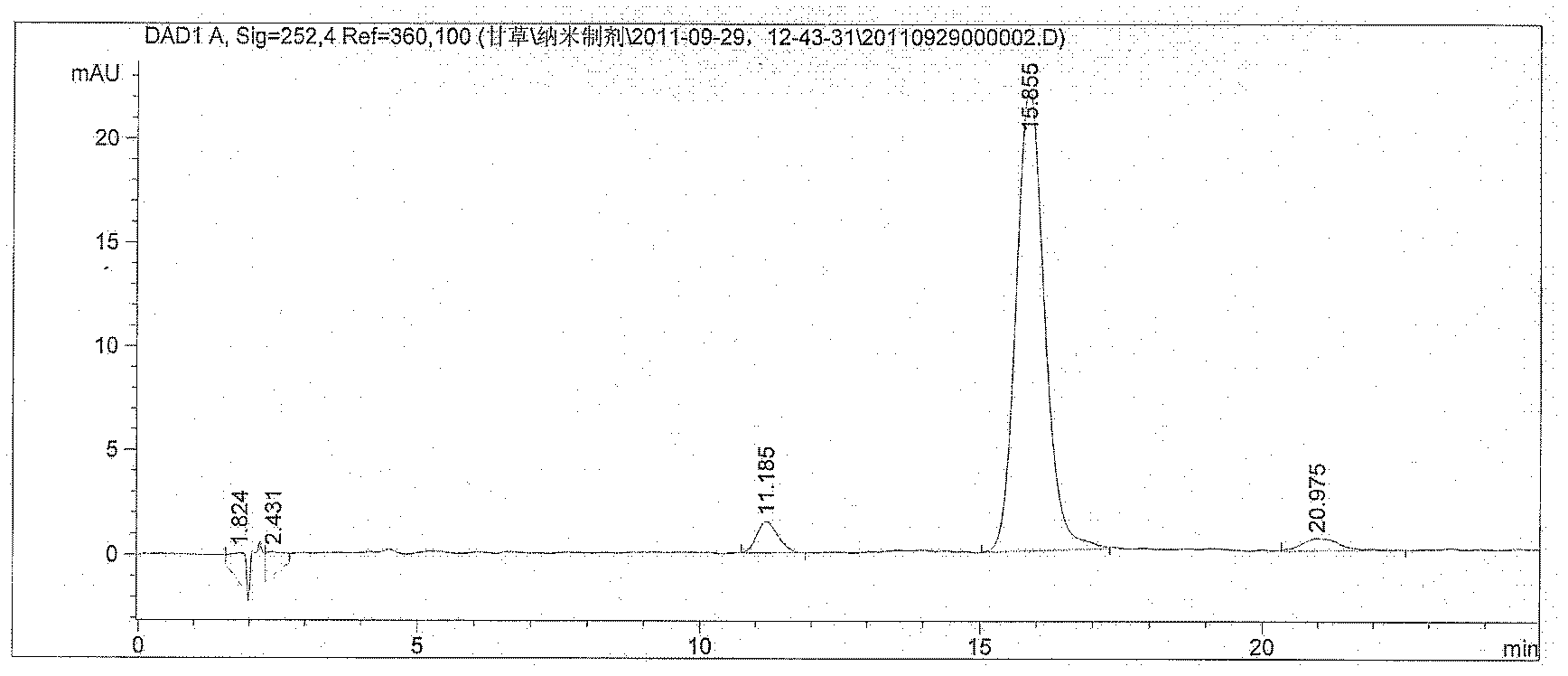
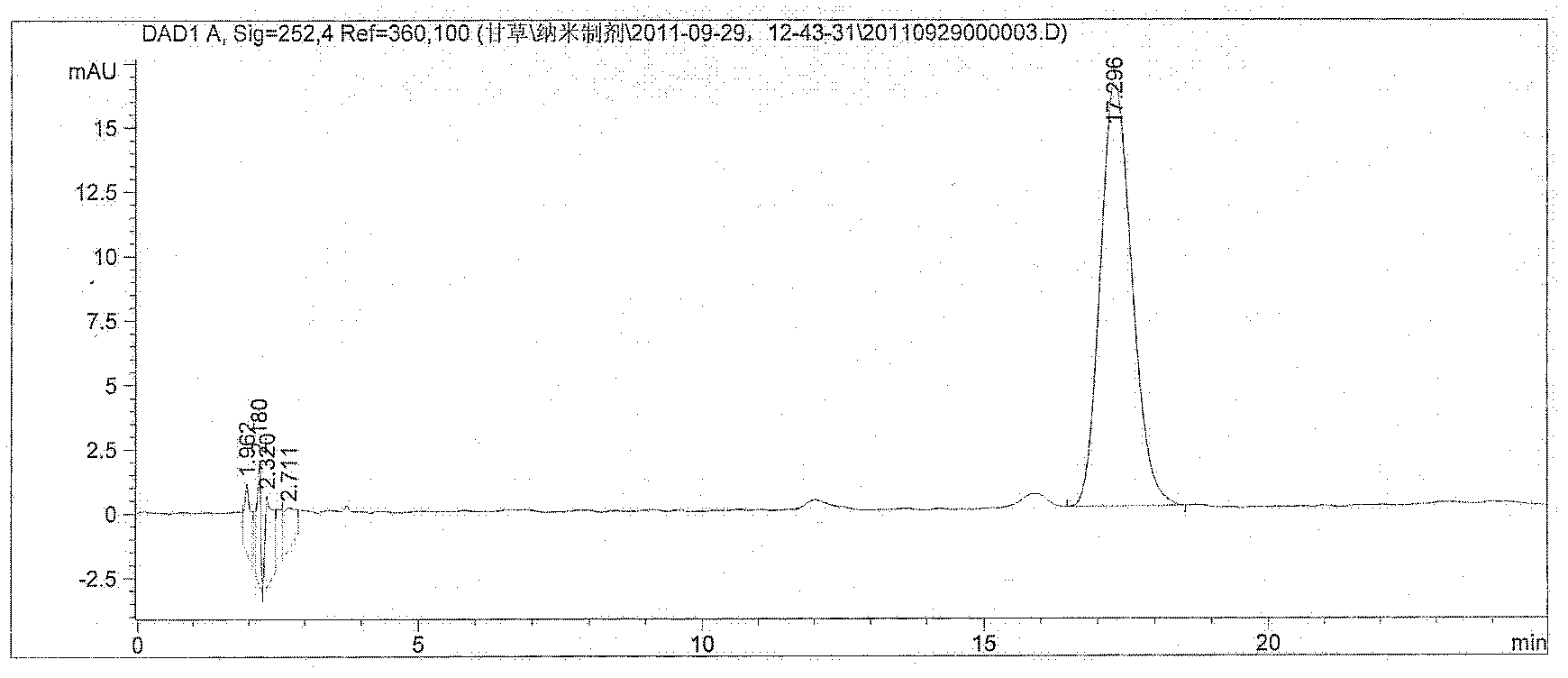
Image
Examples
Embodiment 1
[0036] Take 100g (0.117mol) of diammonium glycyrrhizinate and 500g (15.606mol) of methanol, put them into a reaction tank to mix, stir evenly, add 20g (0.105mol) of p-toluenesulfonic acid, and react for 120 hours under r, t conditions , the crystallization of trans-methyl glycyrrhizinate is complete, filter, wash with methanol, drain, and dry to obtain 60g of high-purity trans-methyl glycyrrhizinate; add 3 times the amount of water to the filtrate, place it overnight, and pour off the supernatant , filtered, washed with appropriate amount of water, drained, and dried to obtain 35g of cis-glycyrrhizic acid (crude product).
[0037] ①Take 60g of trans methyl glycyrrhizinate, add 300ml of sodium hydroxide solution (2mol / L), saponify under r,t conditions until the solution is clear (about 2 hours), add dilute sulfuric acid to adjust the pH to 3.0, and use n-butyl Alcohol extraction to pH 4.0, reduced pressure, evaporated to dryness, and finally add 90% glacial acetic acid to disso...
Embodiment 2
[0045] Take 100g (0.117mol) of diammonium glycyrrhizinate and 350g (10.924mol) of methanol, put them into a reaction tank and mix them, stir evenly, add 8g (0.0816mol) of concentrated sulfuric acid, and react for 72 hours under r, t conditions, trans-glycyrrhizic acid Complete crystallization of methyl ester, filter, wash with methanol, drain, and dry to obtain 61g of high-purity methyl trans-glycyrrhizinate; add 3 times the amount of water to the filter solution, let it stand overnight, pour off the supernatant, filter, and appropriate amount Washed with water, drained and dried to obtain 33g of cis-glycyrrhizic acid (crude product).
[0046] ①Take 61g of trans-methyl glycyrrhizinate, add 305ml of sodium hydroxide solution (2mol / L), saponify under r,t conditions until the solution is clear (about 2 hours), add dilute sulfuric acid to adjust the pH to 3.0, and use n-butyl Alcohol extraction to pH 4.0, reduced pressure, evaporated to dryness, finally added 90% glacial acetic acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com