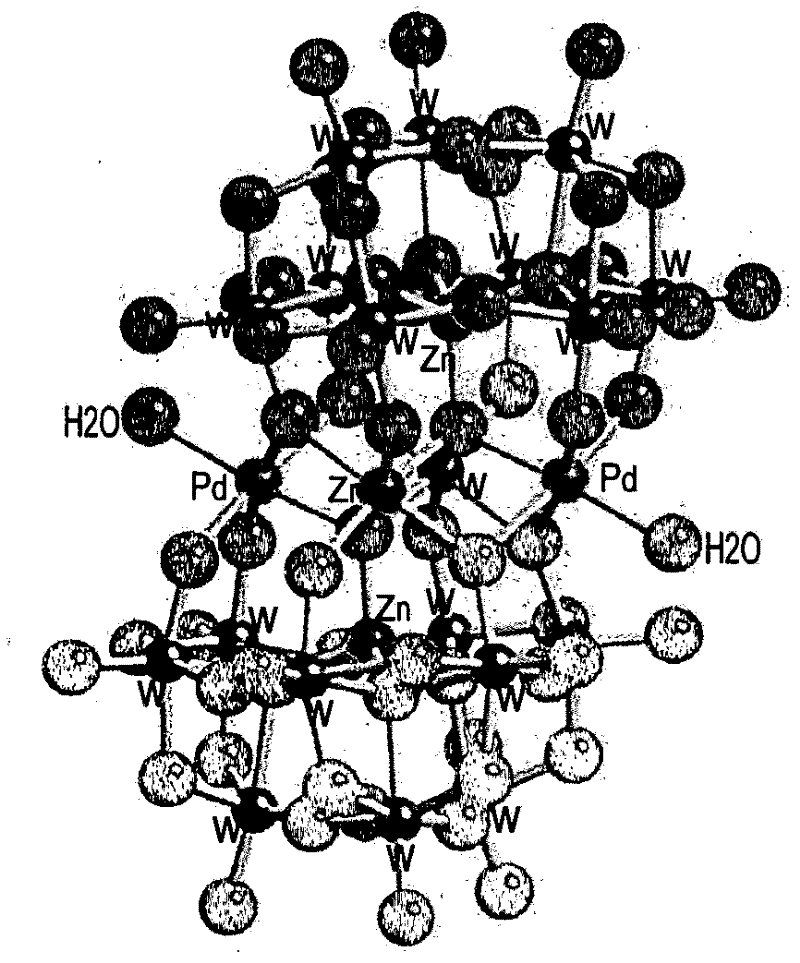
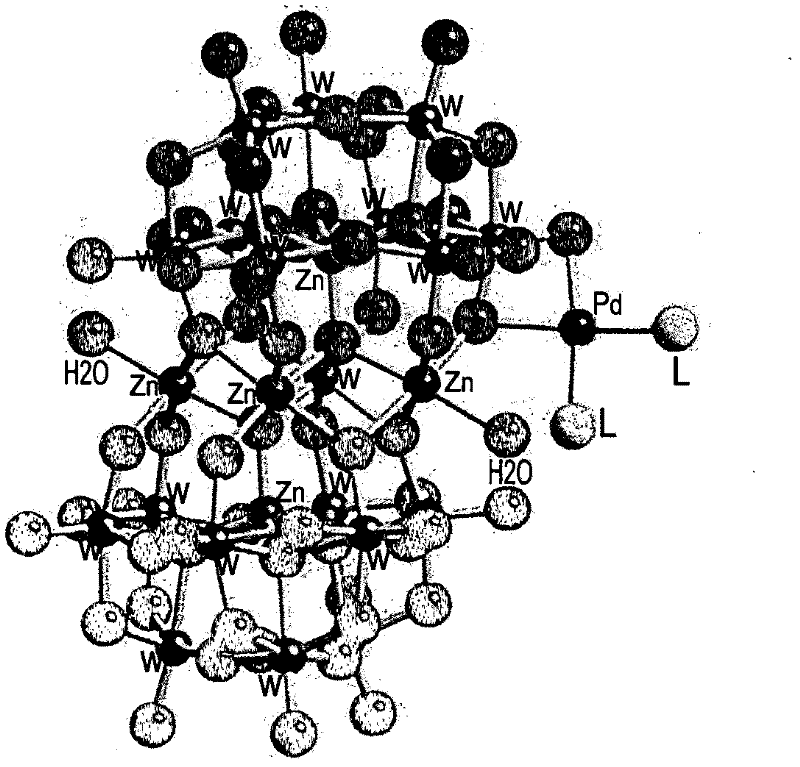
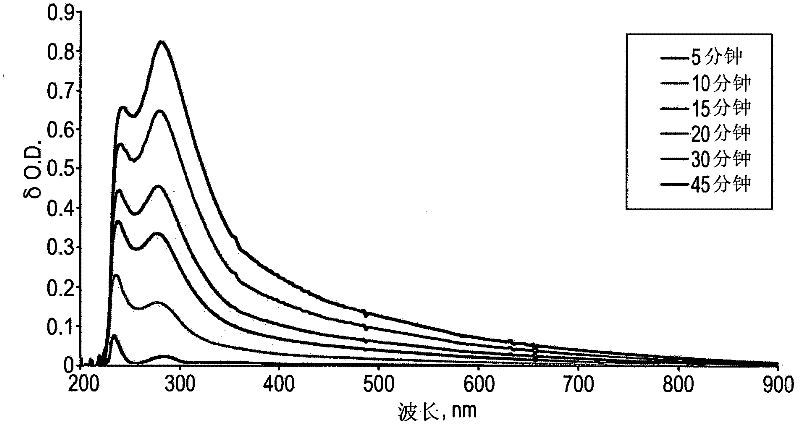
Aerobic oxidation of primary aliphatic alcohols by using noble metal polyoxometalate complexes
A polyoxometalate and complex technology, applied in the field of chemistry, can solve problems such as no description
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Preparation of typical Pd-POM catalyst
[0264] The preparation of a typical NM-POM complex catalyst containing palladium(II) and commonly referred to as Pd-POM is presented below.
[0265] By dropwise adding 1 mmol of parent (mother) polyoxometalate Na dissolved in 10 ml of water 12 [Zn 5 (H 2 O) 2 W 19 o 68 ]·46H 2 O (prepared by the method described by Tourné, C.M. et al. in J. Chem. Soc., Dalton Trans. 1991, 143-155) to 12 mmol of C 16 h 33 (CH 3 ) 3 N + Cl - (25% in water) prepared quaternary ammonium salts of polyoxometalates, Q 12 [Zn 5 (H 2 O) 2 W 19 o 68 ], where Q=C 16 h 33 (CH 3 ) 3 N + .
[0266] The ensuing precipitate was isolated by centrifugation, filtered and dried under vacuum at 80°C overnight, thermogravimetric analysis showed the absence of water.
[0267] Elemental analysis (exp (calculated) %): C, 33.02 (32.76); H, 6.24 (6.03); N, 1.98 (2.01).
[0268] Dissolve 0.167 g (0.5 mmol) in 4 ml CH at 35 °C dropwise 2 Cl 2 Pd[(C...
Embodiment 2
[0275] Catalytic Oxidation of Primary Alcohols Using Pd-POM Catalyst
[0276] The aerobic oxidation of primary alcohols was studied using 1-heptanol as a model substrate. through at 2 bar O 2 A typical reaction was performed by mixing 0.1 mmol of 1-heptanol and 5 μmol of a typical Pd-based catalyst Pd-POM-1 in 1 ml of α,α,α-trifluorotoluene (TFT) at 110 °C. Typically, after 25-30 minutes, the maximum degree of conversion is reached with a high selectivity for n-heptanal. Some minor alcohol peroxidation to n-heptanoic acid and some alcohol oxidation-dehydrogenation of alkanes to β-unsaturated aldehydes were observed, as seen in Scheme 2 below.
[0277] Scenario 2
[0278]
[0279] Using 1:1 PdCl 2 DMSO 2 and Q 12 [Zn 5 (H 2 O) 2 W 19 o 68 ] A similar reaction of the prepared catalyst showed 90% conversion of 1-heptanol with 91% selectivity to heptanal while producing 5% heptanoic acid.
[0280] Several observations and conclusions were drawn from the above experi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com