Method for preparing aldehyde or ketone by using photocatalysis to selectively oxidize primary alcohol or secondary alcohol
A secondary alcohol, selectivity technology, applied in the direction of oxidative preparation of carbonyl compounds, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of low selectivity, achieve the effect of increasing the reaction rate and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
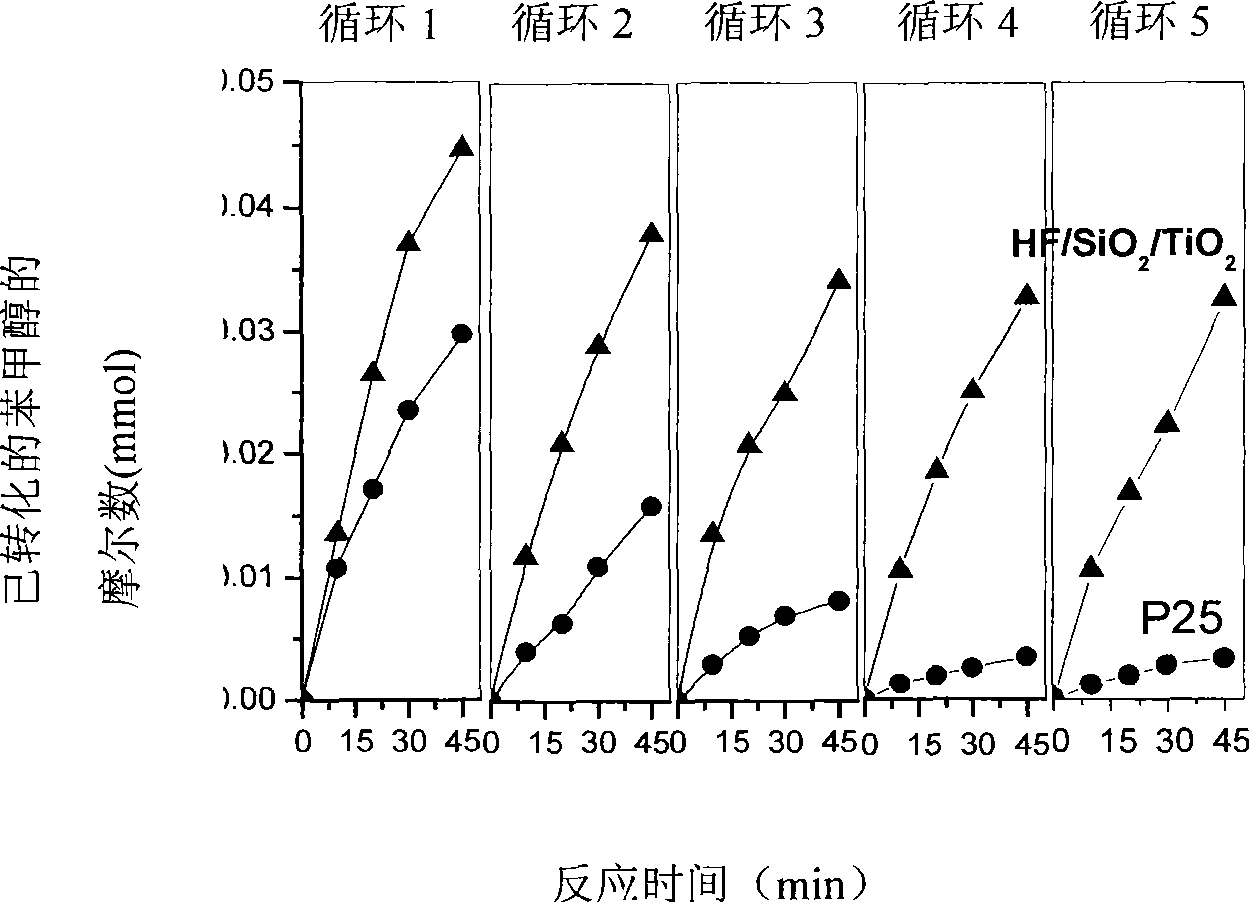
[0034] Preparation of the catalyst: 10 ml of tetrabutyl titanate and a corresponding amount of ethyl silicate (the ratio of Si / Ti atoms is 7%) were added to 300 ml of isopropanol. The solution was kept at 0°C, and distilled water was added dropwise thereto under vigorous stirring. After the tetrabutyl titanate and ethyl silicate were completely hydrolyzed, the white suspension was kept at 80°C for 1 hour. Then centrifugal filtration, the obtained solid was washed three times with ethanol and distilled water respectively. After drying at 100°C, it was calcined at 430°C for 2 hours. Gained sample, take out 0.5g and put in 5ml, 1mol / L HF acid ultrasonic half an hour. Finally, centrifuge filtration to obtain the HF / SiO required for the reaction 2 / TiO 2 Solid-phase catalyst (Si atom in the solid-phase catalyst and Ti atomic number ratio are 7%, and the concentration of HF acid contained on the surface of the solid-phase catalyst is 1×10 -2 mol / g), where TiO 2 Anatase with an...
Embodiment 2
[0052] The preparation method of catalyst is completely with embodiment 1, obtains HF / SiO 2 / TiO 2 solid phase catalyst. where TiO 2 Anatase with an average particle size of 13.5nm, SiO 2 is amorphous.
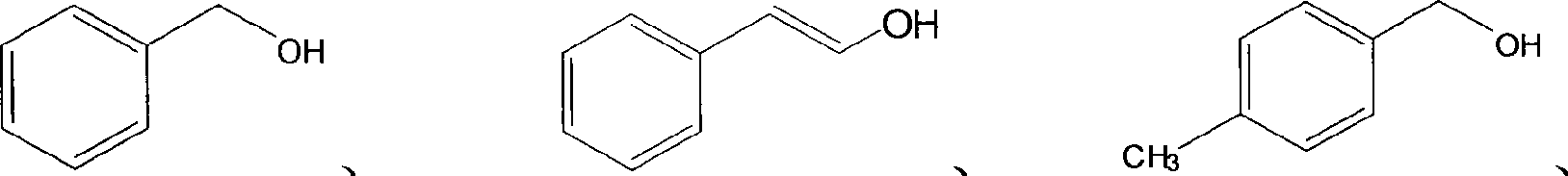
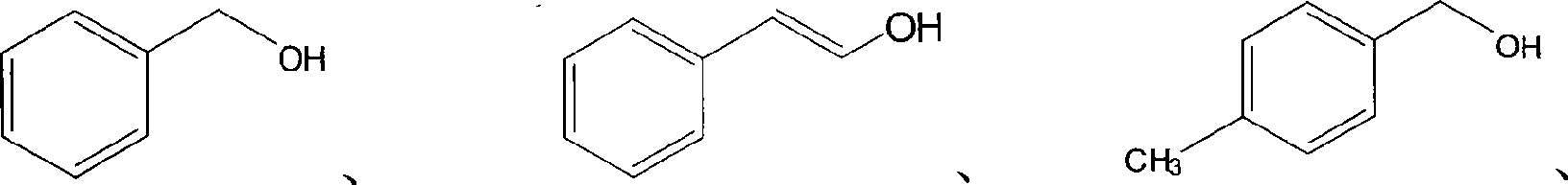
[0053] Preparation of benzaldehyde: add 25mg of HF / SiO to a 15ml glass reactor 2 / TiO 2 and 1.5ml of carbon tetrachloride, acetonitrile or diethyl ether, and then add 0.1mmol of benzyl alcohol. After 0.1MPa oxygen bubbles for half an hour, it is sealed. Turn on the high-pressure mercury lamp (wavelength 220nm-440nm linear discontinuous spectrum, the wavelength that plays a major role is 220nm-420nm), irradiate the reaction for 4 hours under the condition of uniform stirring, turn off the light source, end the reaction, and measure the reaction by GC-MS things and products. The reaction results are shown in Table 3. The influence of several solvents on the selectivity is not much different. On the influence of reaction rate, carbon tetrachloride organic solvent system...
Embodiment 3
[0061] According to the solid-phase catalyst preparation method part in embodiment 1, the ratio of Si / Ti atomic number is prepared as 17% HF / SiO 2 / TiO 2 solid phase catalyst. The concentration of HF acid contained on the surface of the solid phase catalyst is 1×10 -2 mol / g. where TiO 2 Anatase with an average particle size of 13.5nm, SiO 2 is amorphous.
[0062] The preparation of benzaldehyde: the catalyst selects HF / SiO with a Si / Ti atom number ratio of 12%. 2 / TiO 2 Solid-phase catalyst, other reaction conditions and reaction steps are with the preparation part of benzaldehyde in embodiment 2. After the irradiation reaction for 4 hours, the light source was turned off, the reaction was terminated, and the reactants and products were determined by GC-MS. The conversion rate of benzyl alcohol is 93%, and the selectivity of forming benzaldehyde is 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com