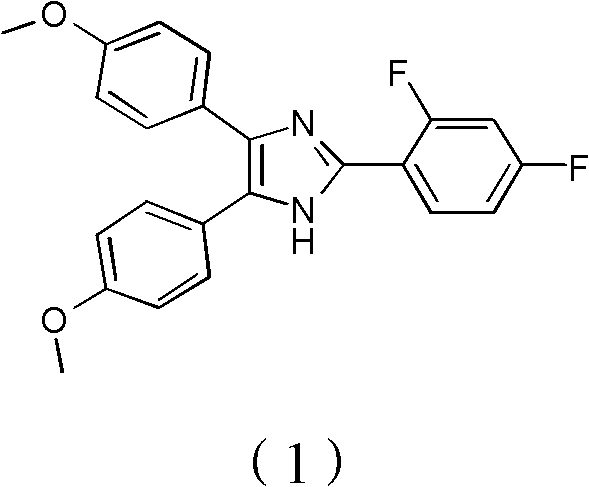
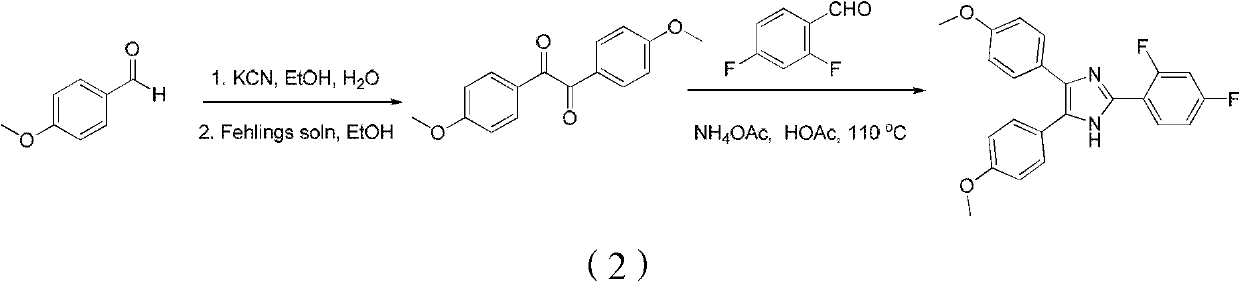
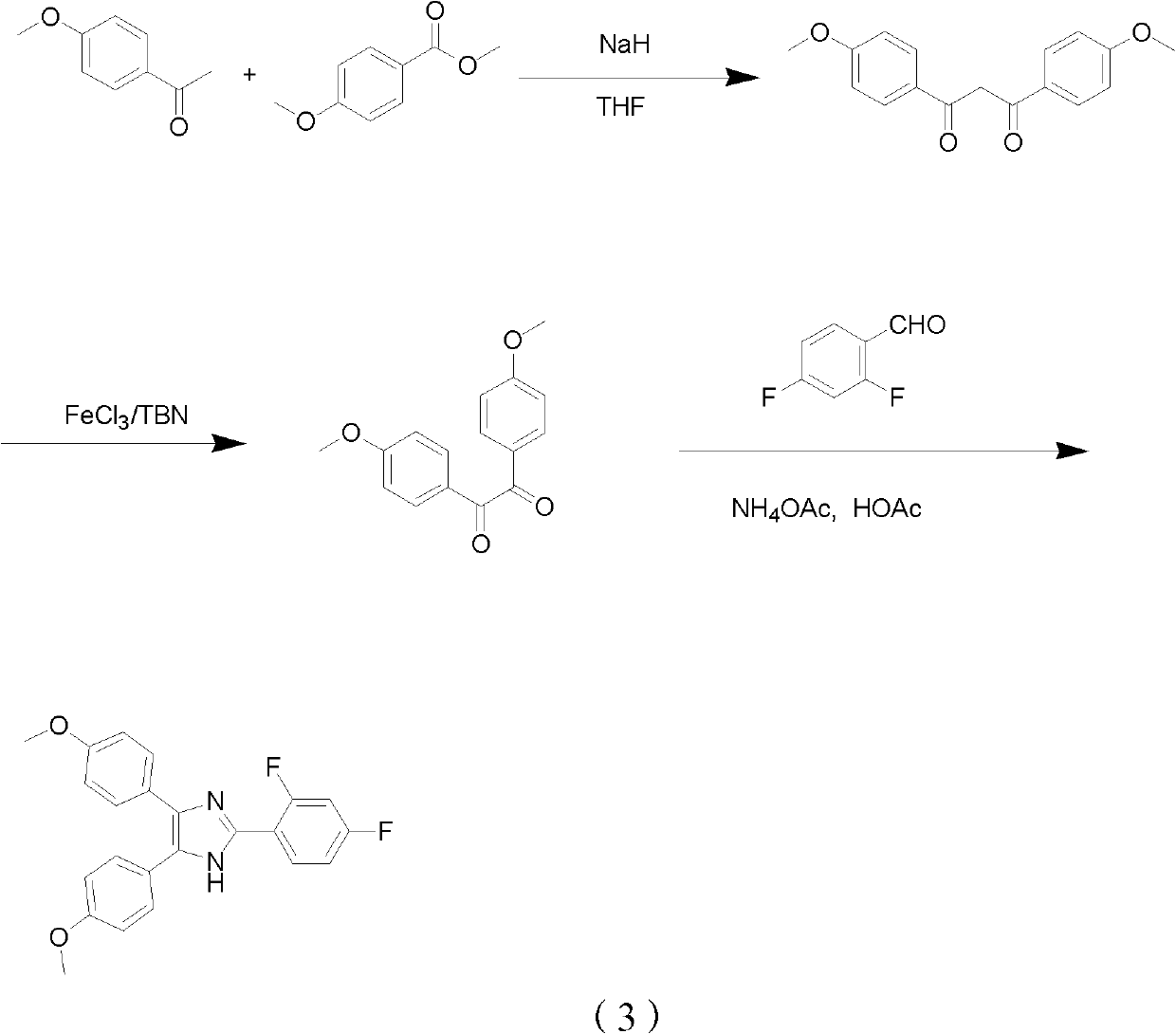
Method for synthetizing fenflumizole
A technology of imidazole and difluorophenyl, which is applied in the field of organic chemical synthesis, can solve problems such as great danger, and achieve the effects of low cost, avoidance of use, and safe application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1,1, the synthesis of 3-bis(4-methoxybenzene)-1,3 diketone
[0030]
[0031] Sodium hydride (60%, 4.0 g, 100 mmol) and dry tetrahydrofuran (50 mL) were added to a 250 mL one-necked flask. After stirring in ice bath for 20 min, methyl 4-methoxybenzoate (7.3 g, 44 mmol) was added, and then 4-methoxyacetophenone (6.0 g, 40 mmol) was added dropwise. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred for 12 hours. After TLC (thin-layer chromatography) showed that the reaction was complete, water was added dropwise to the reaction system to quench the reaction, and dilute hydrochloric acid was added to adjust the pH to about 3, extracted three times with ethyl acetate, and dried over anhydrous sodium sulfate. The organic solvent was spin-off on a rotary evaporator, and the obtained crude product was recrystallized from a mixed solvent of petroleum ether and ethyl acetate to obtain 8.0 g of 1,3-bis(4-methoxybenzene)-1,3-dione a...
Embodiment 2
[0042] Step 1,1, the synthesis of 3-bis(4-methoxybenzene)-1,3 diketone
[0043] Sodium hydride (60%, 3.2g, 80mmol) and dry tetrahydrofuran (50mL) were added into a 250mL single-necked flask, and after stirring for 20min under ice bath, methyl 4-methoxybenzoate (3.3g, 20mmol) was added, and then Then 4-methoxyacetophenone (6.0 g, 40 mmol) was added dropwise. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred for 12 hours. After TLC showed that the reaction was complete, water was added dropwise to the reaction system to quench the reaction, and the pH was adjusted to about 3 by adding dilute hydrochloric acid, extracted three times with ethyl acetate, and dried over anhydrous sodium sulfate. The organic solvent was spin-off on a rotary evaporator, and the obtained crude product was recrystallized from a mixed solvent of petroleum ether and ethyl acetate to obtain 3.9 g of 1,3-bis(4-methoxybenzene)-1,3-dione as a yellow solid. rate of 68...
Embodiment 3
[0049] Step 1,1, the synthesis of 3-bis(4-methoxybenzene)-1,3 diketone
[0050] Sodium hydride (60%, 6.4g, 160mmol) and dry tetrahydrofuran (50mL) were added into a 250mL single-necked flask, and after stirring for 20min under ice bath, methyl 4-methoxybenzoate (8.0g, 48mmol) was added, and then Then 4-methoxyacetophenone (6.0 g, 40 mmol) was added dropwise. After the dropwise addition was completed, the temperature was raised to 60° C. and stirred for 12 hours. After TLC showed that the reaction was complete, water was added dropwise to the reaction system to quench the reaction, and the pH was adjusted to about 3 by adding dilute hydrochloric acid, extracted three times with ethyl acetate, and dried over anhydrous sodium sulfate. The organic solvent was spin-off on a rotary evaporator, and the obtained crude product was recrystallized from a mixed solvent of petroleum ether and ethyl acetate to obtain 8.3 g of 1,3-bis(4-methoxybenzene)-1,3-dione as a yellow solid. rate of 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com