Scutellarin carbamate derivative, preparation method and use thereof
A technology of carbamates and scutellarin aglycone is applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, which can solve the problems of serious toxic and side effects, poor solubility of scutellarin, and short half-life in vivo.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
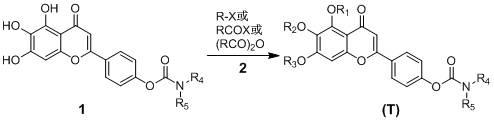
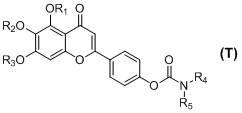
[0034] 5,6,7-trimethoxy-4'-( N - Preparation of tetrahydropyrrolecarboxy)-flavone (T-1)
[0035] Add 5,6,7-trihydroxy-4'-( N -Tetrahydropyrrole carboxyloxy)-flavone 1.0 mmol, acetone 30 ml, anhydrous potassium carbonate 12.0 mmol and dimethyl sulfate 10.0 mmol, heated and refluxed and stirred for 24 hours (the reaction process was tracked by TLC); after the reaction, Filtrate while hot, wash the filter cake with a small amount of acetone, evaporate the filtrate to remove the solvent under reduced pressure, add 30 ml of ice water to the residue, and filter with suction. The obtained crude product is recrystallized from ethanol to obtain 0.39 g of a yellow powder solid, with a yield of 92.0%; HR- TOFMS (+Q) m / z :426.1551 ([C23 h 23 NO 7 +H] + Calculated value: 426.1553).
Embodiment 2
[0037] 5-hydroxy-6,7-dimethoxy-4'-( N -tetrahydropyrrolecarboxy)-flavone (T-2) and 5,7-dihydroxy-6-methoxy-4′-( N - Preparation of tetrahydropyrrolecarboxy)-flavone (T-3)
[0038] Add 5,6,7-trihydroxy-4'-( N -Tetrahydropyrrolecarboxy)-flavone 1.0 mmol, acetone 30 ml, anhydrous potassium carbonate 3.0 mmol and dimethyl sulfate 2.9 mmol, heat up to 40-45°C and keep stirring for 18 hours (track the reaction process with TLC) After the reaction, filter while it is hot, wash the filter cake with a small amount of acetone, evaporate the filtrate to remove the solvent under reduced pressure, add 30 ml of ice water to the residue, and filter with suction. The obtained crude product is dried and purified by column chromatography (eluent: chloroform- Methanol=50:1 v / v), to get 5-hydroxy-6,7-dimethoxy-4′-( N -tetrahydropyrroloyloxy)-flavone yellow powder solid 0.186 g, yield 45.2%; HR-TOFMS (+Q) m / z :412.1399 ([C 22 h 21 NO 7 +H] + Calculated: 412.1396); and 5,7-dihydroxy-6-m...
Embodiment 3
[0040] 5,6,7-trimethoxy-4'-( N,N - Preparation of dimethylcarbamoyloxy)-flavone (T-4)
[0041] The operating process is the same as in Example 1, except that 5,6,7-trihydroxyl-4'-( N -Tetrahydropyrrolecarboxy)-flavone with 5,6,7-trihydroxy-4'-( N,N -Dimethylcarbamoyloxy)-flavone instead, dimethyl sulfate is replaced by iodomethane to give 5,6,7-trimethoxy-4'-( N,N -Dimethylcarbamoyloxy)-flavone yellow powder solid, yield 90.0%; HR-TOFMS (+Q) m / z :400.1395 ([C 21 h 21 NO 7 +H] + Calculated value: 400.1396).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com