Stable moxifloxacin hydrochloride compound
A technology of moxifloxacin hydrochloride and its compound, which is applied in the field of pharmaceuticals in the field of medicine, and can solve the problems of SFDA not approving the production of domestic manufacturers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
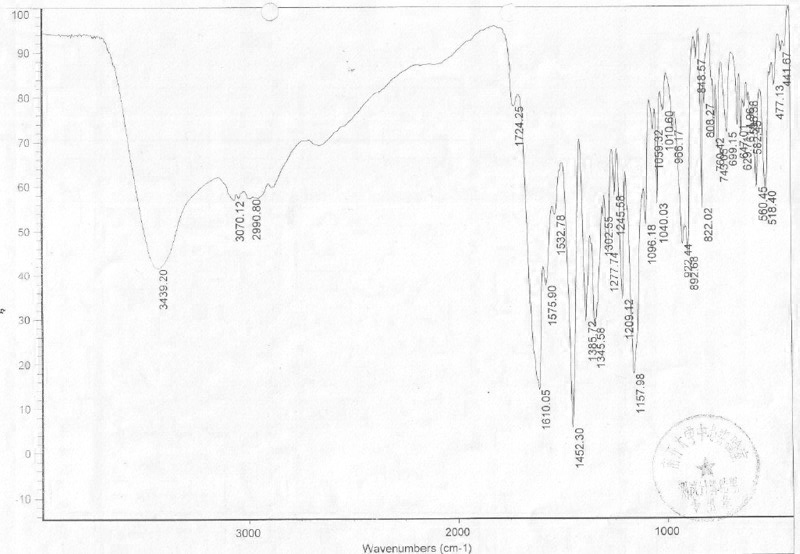
[0078] In a 5000ml reaction bottle equipped with stirring, thermometer and condenser, add 400g of moxifloxacin hydrochloride and 4000ml of ethanol-acetonitrile-water (6:2:2) mixture, start stirring, and heat up to 55°C-60°C ℃, until all dissolved, add 1 gram of potassium nitrate, filter while hot, naturally cool the filtrate to 30 ℃, add 6mol / L hydrochloric acid dropwise until the pH is 2.0, stir at room temperature for 2 hours, then stir at -5 ℃ for 2 hours , precipitated crystals, filtered, washed with ethanol, and dried at 45°C to obtain 371.8 grams of high-purity white crystals of the above-mentioned moxifloxacin hydrochloride compound. As determined by the Karl Fischer method, it contains 3.96% (weight percent) of water.
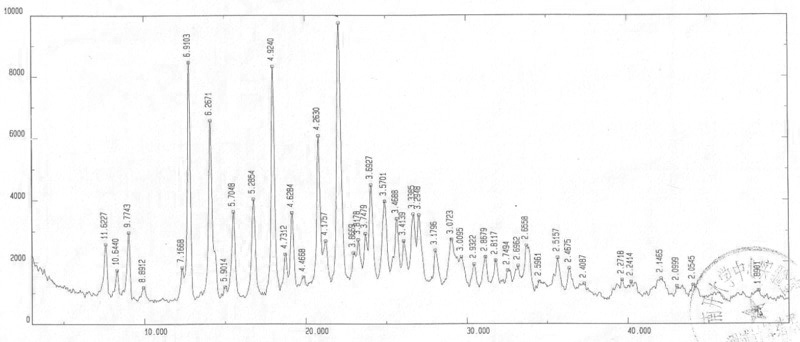
[0079] Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° .
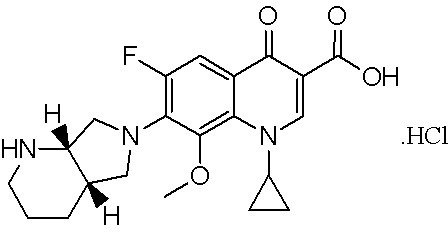
[0080] The structural formula (I) of the compound is shown in figure 1 .
[0081] The X-ray diffraction pattern of ...
Embodiment 2
[0085] Granules containing moxifloxacin hydrochloride compound
[0086] Prescription: 400 grams of moxifloxacin hydrochloride compound as moxifloxacin, 480 grams of lactose, 60 grams of crospovidone, 160 grams of hydroxypropyl methylcellulose, appropriate amount of distilled water, made into 1000 bags.
[0087] Process: Moxifloxacin hydrochloride compound is mixed with various auxiliary materials, passed through an 80-mesh sieve, made into a soft material with distilled water, granulated, dried, and then packed into granules.
Embodiment 3
[0089] Capsules containing the moxifloxacin hydrochloride compound
[0090] Prescription: moxifloxacin hydrochloride compound is calculated as moxifloxacin 400 grams, pregelatinized starch 150 grams, made into 1000 capsules.
[0091] Process: Moxifloxacin hydrochloride compound and starch aqueous solution are moistened, mixed evenly, sieved and granulated, dried at 60°C, granulated, and filled into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com