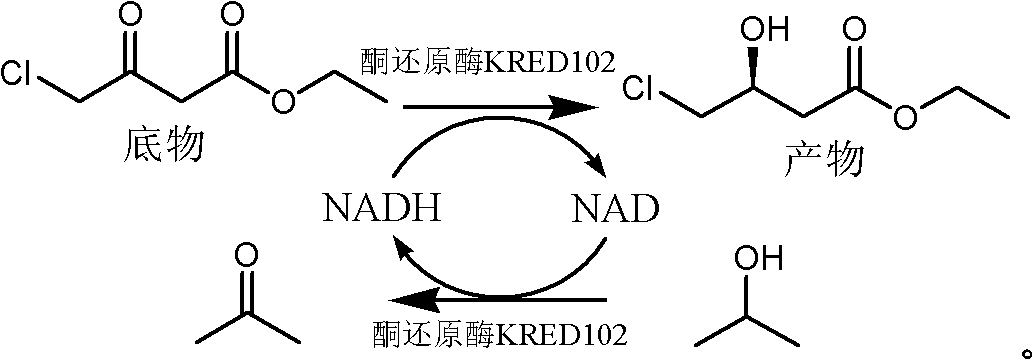
(S)-4-chloride-3-ethyl 3-hydroxybutyrate biological preparation method
The technology of ethyl hydroxybutyrate and ethyl carbonyl butyrate is applied in the field of biological preparation of ethyl-4-chloro-3-hydroxybutyrate, can solve the problems of expensive cofactors, complicated operation and the like, achieves simple operation, Improves the effect of low substrate concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
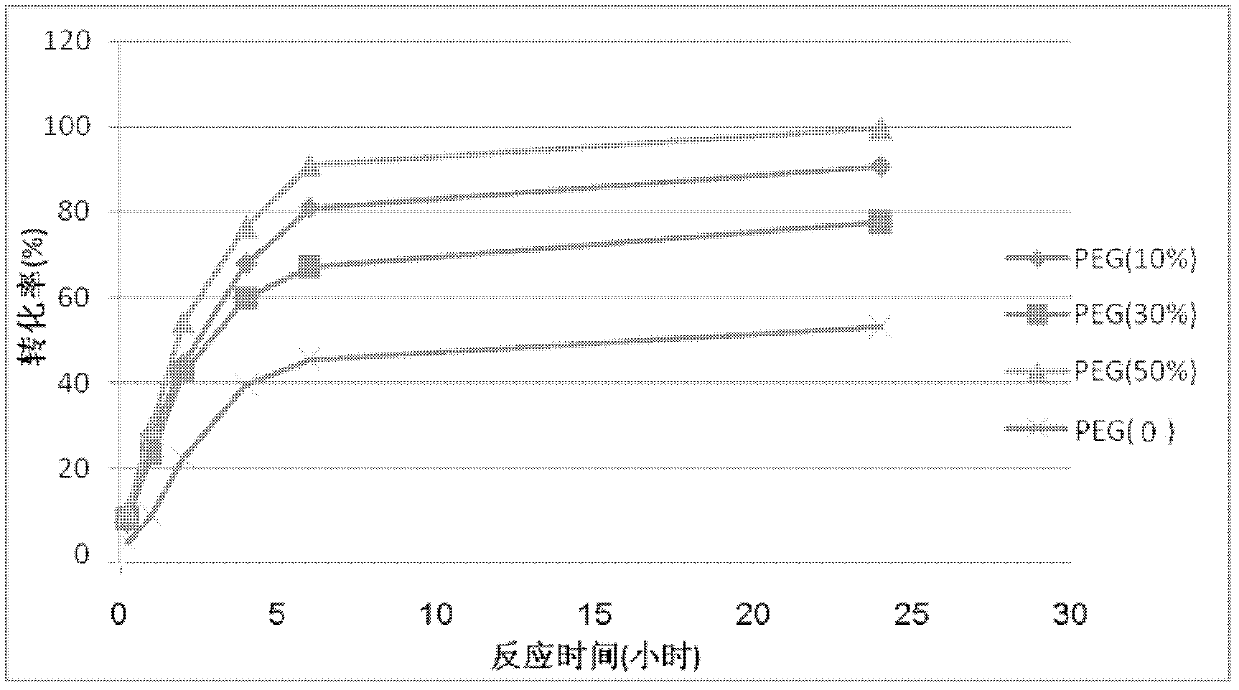
[0022] Take four 20ml three-necked flasks, add 2.37ml phosphate buffer solution (100mM, pH 6.5) to each, and add 1.33ml of substrate 4-chloro-3-carbonyl butyrate ethyl ester, isopropanol 1.3ml, ketone Reductase KRED (commercially purchased) 1020.067g, in three flasks wherein, add respectively the polyethylene glycol 400 of 10%, 30% and 50% of reaction system total volume (10mL), finally add phosphate in each flask The buffer solution was added to 10 ml, and the reaction was stirred at 200 rpm at 30° C., and the conversion rate of the reaction was monitored by gas chromatography. The reaction conversion rate in each flask varies with time as shown in figure 1 shown. From figure 1 Visible, when polyethylene glycol add-on is 50%, the conversion rate of reaction is maximum.
Embodiment 2
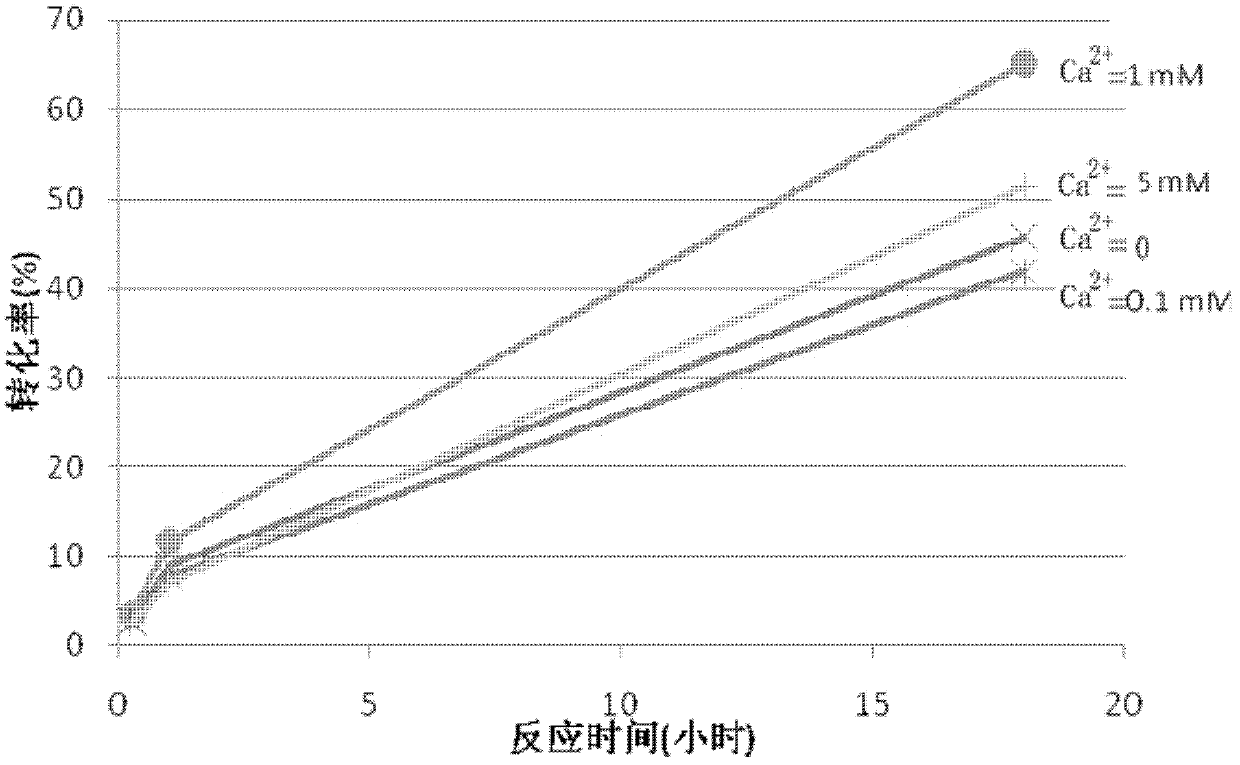
[0024] Take four 20ml three-neck flasks, add 2.37ml phosphate buffer solution (100mM, pH 6.5) respectively, and add substrate 4-chloro-3-carbonyl butyric acid ethyl ester 1.33ml, isopropanol 1.3ml respectively successively, Ketoreductase KRED102 (commercially purchased) 0.067g and polyethylene glycol 4005ml, also add the anhydrous calcium chloride of 0.1mM, 1mM and 5mM respectively in three flasks wherein, at 30 ℃, 200rpm stirring reaction, utilize The conversion of the reaction was monitored by gas chromatography. The reaction conversion rate in each flask varies with the reaction time as shown in figure 2 shown. From figure 2 It can be seen that when the amount of calcium ions added is 1mM, the conversion rate of the reaction is the largest.
Embodiment 3
[0026] Add 2.37ml of phosphate buffered saline solution (100mM, pH 6.5) into a 20mL three-necked flask, add substrate 1.33ml of ethyl 4-chloro-3-carbonylbutyrate, 1.30ml of isopropanol, ketoreductase KRED102 (commercial Purchase) 0.02g, polyethylene glycol 4005ml, anhydrous calcium chloride 1mM, under 30 ℃, 200rpm stirring reaction, utilize gas chromatography to monitor the conversion rate of reaction. After 24 hours the conversion reached 85.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com