Application of lincomycin in medicine for treating meniscus injury
A technology of lincomycin and meniscus, applied in the direction of drug combination, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of no reliable treatment and repair methods for meniscus, and achieve the effect of functional recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
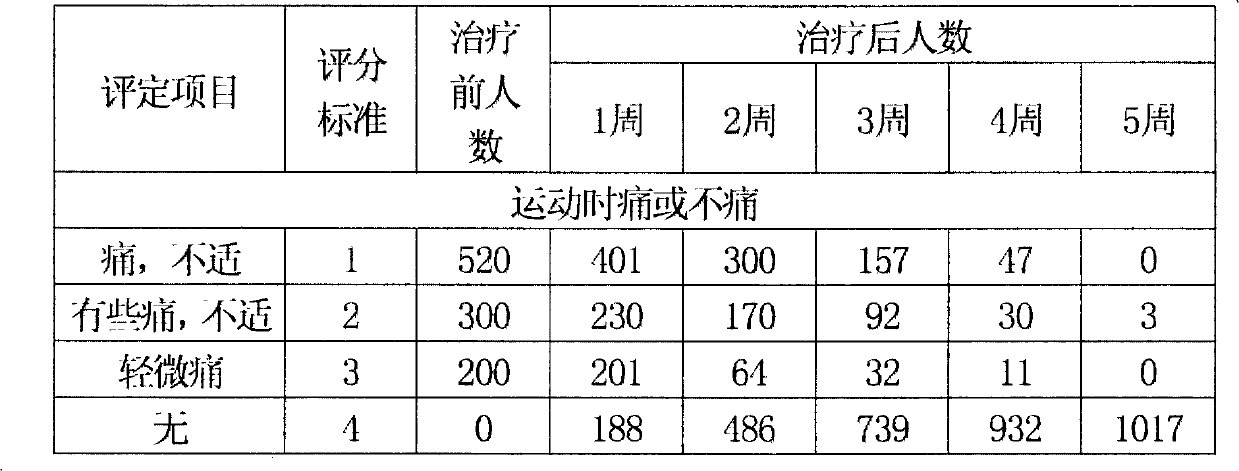
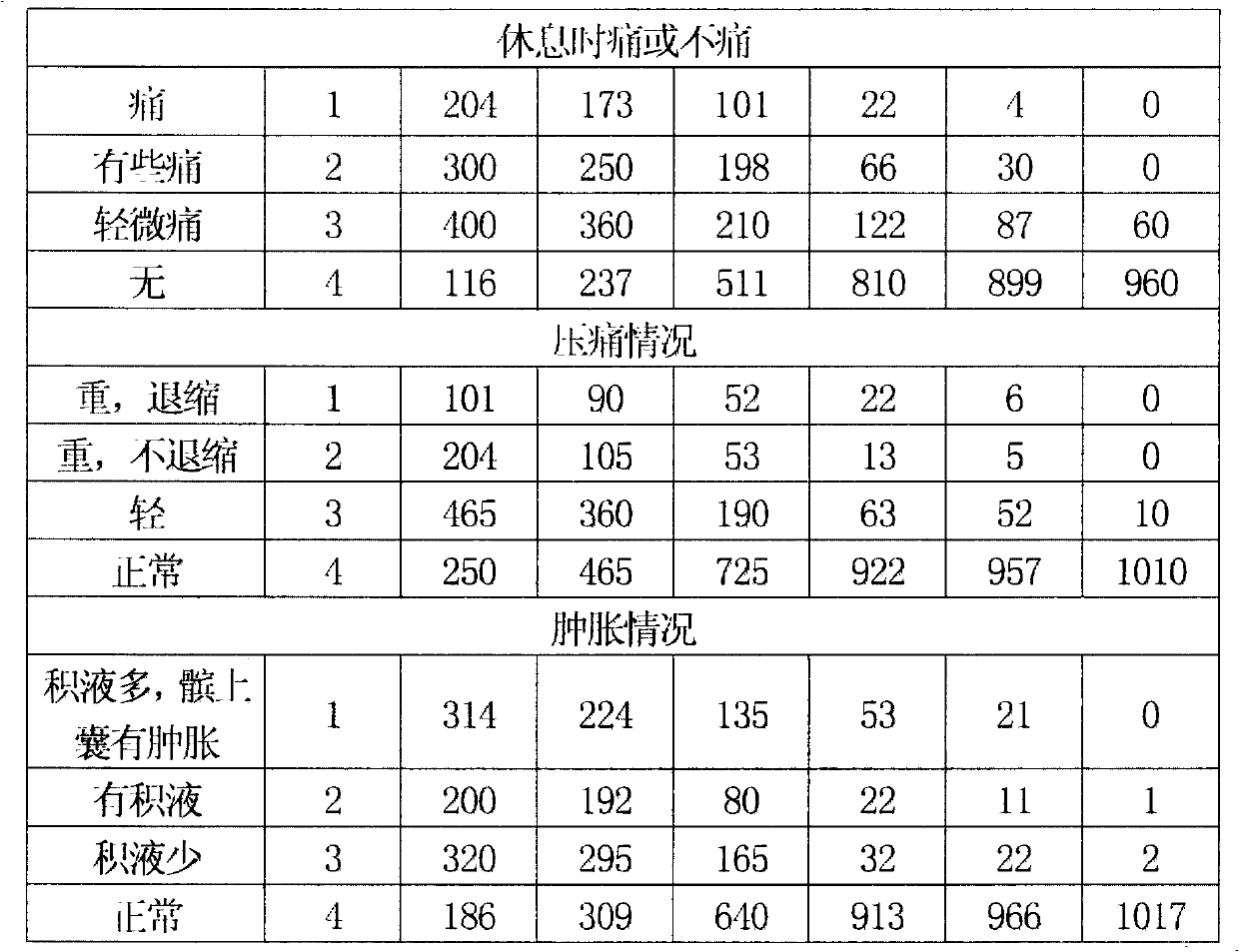
Image
Examples
Embodiment 1
[0064] Drugs used:
[0065] The third generation display 20ml×1 tube;
[0066] 2% lidocaine needle 5ml×1 stick;
[0067] Sodium hyaluronate 2ml×2 sticks, produced by Shanghai Qisheng Biological Preparations Co., Ltd.;
[0068] Lincomycin 0.3g×2 sticks;
[0069] Dexamethasone 5mg×2 sticks;
[0070] 0.9% normal saline 500ml×4 bottles;
[0071] Lincomycin is relatively common, and the quality of each manufacturer has little difference.
[0072] Puncture method and surgical steps:
[0073] Routine local skin disinfection with povidone iodine, and routine draping. The medial midpoint of the patella was selected as the puncture point, and local anesthesia was performed with 2% lidocaine. Make a skin incision about 5mm long at the puncture point, puncture into the suprapatellar bursa through the incision, and inject a small amount of diluted contrast medium to confirm that the puncture needle has entered the suprapatellar bursa. Adjust the direction of the puncture needle to ...
Embodiment 2
[0076] Drugs used:
[0077] The third generation display 20ml×1 tube;
[0078] 2% lidocaine needle 5ml×1 stick;
[0079] Sodium hyaluronate 2ml×2 sticks, produced by Shanghai Qisheng Biological Preparations Co., Ltd.;
[0080] Lincomycin 0.3g×3 sticks;
[0081] Dexamethasone 5mg×2 sticks;
[0082] 0.9% normal saline 500ml×4 bottles;
[0083] Lincomycin is relatively common, and the quality of each manufacturer has little difference.
[0084] Puncture method and surgical steps:
[0085] Disinfection, puncture and other processes are the same as in Example 1, and then the joint fluid is completely sucked out and sent for inspection. Then lincomycin and dexamethasone were injected sequentially and slowly through the catheter, and sodium hyaluronate was injected after an interval of 1 minute. Finally, 2% lidocaine was used to flush the residual sodium hyaluronate in the catheter into the joint cavity. Extubation after confirming that there is no residual sodium hyaluronate ...
Embodiment 3
[0088] Drugs used:
[0089] The third generation display 20ml×1 tube;
[0090] 2% lidocaine needle 5ml×1 stick;
[0091] Sodium hyaluronate 2ml×3 sticks, produced by Bausch & Lomb Freda Pharmaceutical Co., Ltd., trade name Speight;
[0092] Lincomycin 0.3g×3 sticks;
[0093] Dexamethasone 5mg×3 sticks;
[0094] 0.9% normal saline 500ml×4 bottles;
[0095] Lincomycin is relatively common, and the quality of each manufacturer has little difference.
[0096] Puncture method and surgical steps:
[0097] Disinfection, puncture and other processes are the same as in Example 1, and then the joint fluid is completely sucked out and sent for inspection. Then inject lincomycin sequentially and slowly through the catheter, inject dexamethasone at intervals of 3 minutes, and inject sodium hyaluronate at intervals of 3 minutes; finally use 2% lidocaine to flush the residual sodium hyaluronate in the catheter into the joint cavity . Extubation after confirming that there is no resid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com