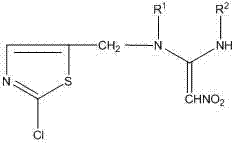
Anabasine compound, preparation method and application of nabasine compound
A technology of neonicotinoids and compounds, applied in the field of organic compounds and their preparation, can solve problems such as low insecticidal activity, environmental hazards, and use restrictions, and achieve broad insecticidal spectrum, obvious insecticidal effect, and insecticidal activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
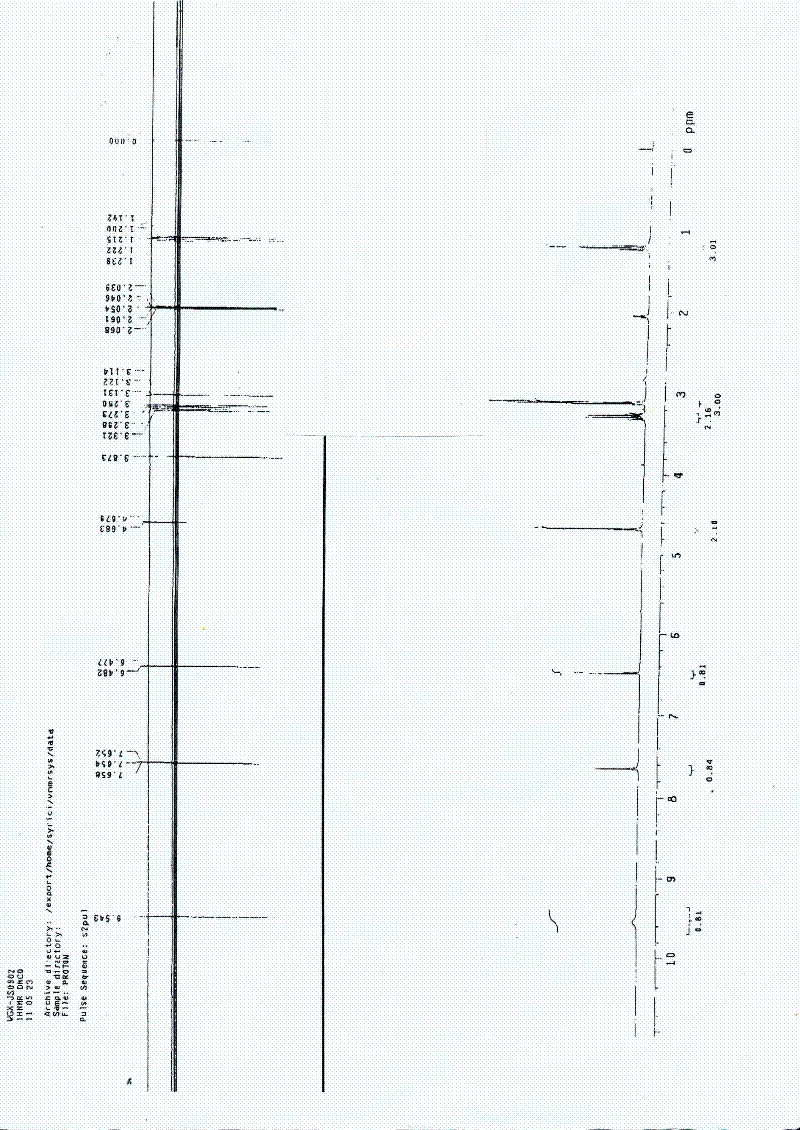
[0029] The neonicotinoid compound in this example is (E)-N-[(2-chloro-thiazole)methyl]-N-ethyl-N'-methoxy-2-nitroethylenediamine. Its structural formula is as follows:
[0030]
[0031] The preparation method of the neonicotinoid compound has the following steps:
[0032] ①Add 33.6g of 2-chloro-5-chloromethylthiazole (0.2mol) and 100mL of chloroform into a 500mL four-necked bottle, then add 0.5g~1.0g of tetrabutylammonium bromide and 38.6g of 70wt% ethylamine aqueous solution (0.6mol), heated to reflux for 4h~5h under stirring. Sampling gas chromatographic analysis until 2-chloro-5-chloromethylthiazole has been completely converted, cooled to 20°C, added 100mL of water, stirred for 20min, stood still, separated the oil layer, extracted the water layer with chloroform three times, combined the oil layer, washed with water To neutrality, 33.45g of N-(2-chloro-5-thiazole)methyl-N-ethylamine was obtained after precipitation, the content was 95%, and the yield was 90%.
[003...
Embodiment 2)
[0036] This example is basically the same as Example 1, except that in step ② triethylamine is used instead of potassium carbonate, resulting in 41.05 g of (E)-N-[(2-chloro-thiazole)methyl]-N- Ethyl-N'-methoxy-2-nitrovinylidenediamine, the content is 97%, and the yield is 72%.
Embodiment 3)
[0038] Step 1. of the present embodiment is the same as Embodiment 1, and step 2. is:
[0039] Add 39.3g of 1,1,1-trichloronitroethane (0.22mol) and 100mL of chloroform into a 500mL four-neck flask, drop the temperature to a reaction solution temperature of 0-5°C and add 35.5g concentration It is a 40wt% potassium carbonate aqueous solution, stir for 2 hours after the addition, and then dropwise add 35.3g of N-(2-chloro-5-thiazole)methyl-N-ethylamine (0.2mol) dichloro Methane solution, stirred at 10°C for 3h after addition. Sampling liquid chromatographic analysis until N-(2-chloro-5-thiazole)methyl-N-ethylamine is completely converted, at this temperature, 53g of methoxyamine hydrochloride aqueous solution (0.25mol) with a concentration of 40wt% is added dropwise , and then neutralized with a KOH solution with a concentration of 30wt% to a pH of 9, and reacted at this temperature for 1 hour, and then reacted at ambient temperature (15-25°C) for 4 hours, stood still, separate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com