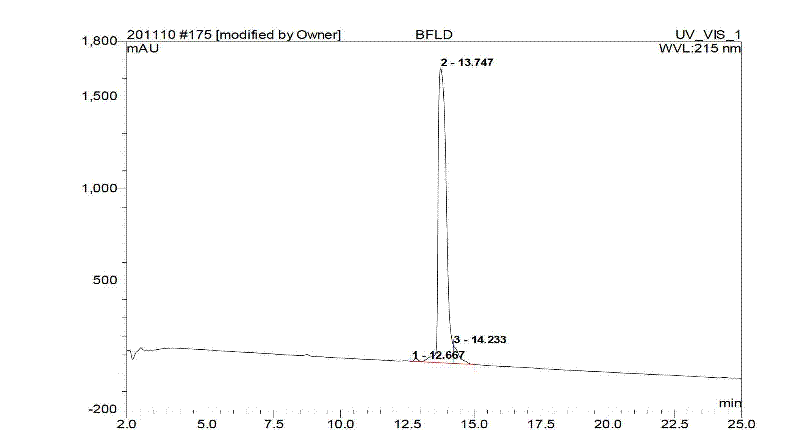
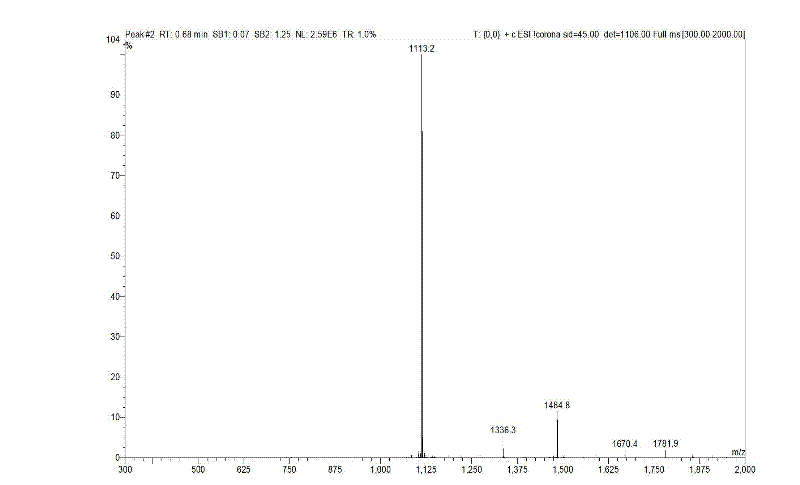
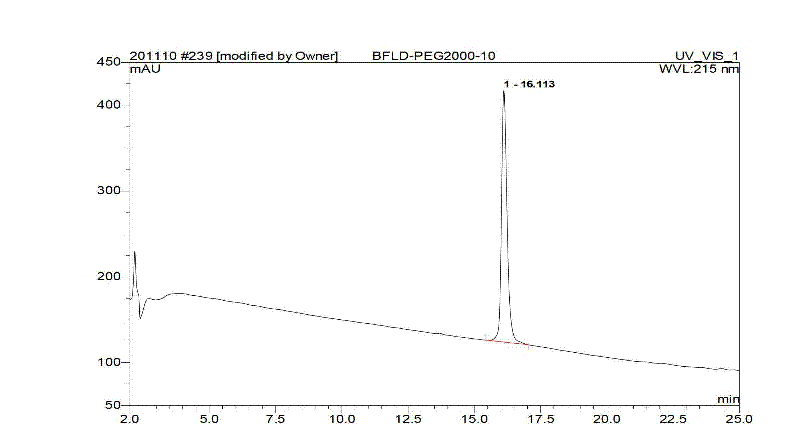
Bivalirudin-polyethylene glycol compound
A technology of polyethylene glycol and bivalirudin, applied in drug combinations, medical preparations of non-active ingredients, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 [Cys (mPEG 2000 -MAL)] 7 - Preparation of BVLD
[0059] Weigh mPEG 2000 -OH 20g (10mmol) was placed in a reaction flask, dichloromethane 50mL was added, and the solid was dissolved, then triethylamine 7.5mL (50mmol) and p-toluenesulfonyl chloride 9.5g (Ts-Cl, 50mmol) were added, and the reaction was stirred at room temperature. After the reaction is complete as detected by TLC, remove the solvent by rotary evaporation, add 50 mL of anhydrous ether to precipitate a solid, add water to dissolve the solid, separate the liquid, separate the water layer, wash twice with 50 mL of anhydrous ether, and then wash the water phase with 75 mL of dichloromethane Extracted twice, collected the organic phase and dried overnight. The solvent was removed by rotary evaporation, and anhydrous diethyl ether was added to precipitate a solid. mPEG after drying 2000 -OTs 14.2g, yield 71%.
[0060] Dissolve mPEG-OTs 14g (7mmol) in 30mL DMF, add phthalimide potassium salt 3.8...
Embodiment 2
[0066] Embodiment 2[Cys(mPEG 5000 -MAL)] 7 - Preparation of BVLD
[0067] Weigh mPEG 5000 -OH 25g (5mmol) was placed in a reaction flask, dichloromethane 100mL was added, and the solid was dissolved, then triethylamine 7.5mL (5mmol) and p-toluenesulfonyl chloride 9.5g (Ts-Cl, 50mmol) were added, and the reaction was stirred at room temperature. After the TLC detection reaction is complete, remove the solvent by rotary evaporation, add 150 mL of anhydrous ether to precipitate a solid, add water to dissolve the solid, separate the liquid, separate the water layer, wash twice with 100 mL of anhydrous ether, and then wash the water phase with 150 mL of dichloromethane Extracted twice, collected the organic phase and dried overnight. The solvent was removed by rotary evaporation, and anhydrous ether was added to precipitate a solid, which was filtered and dried to obtain mPEG 5000 -OTs 21.6g, yield 86%.
[0068] mPEG 5000 -OTs 20g (4mmol) was dissolved in 30mL DMF, phthalimid...
Embodiment 3
[0072] Embodiment 3 [Cys (mPEG 10000 -MAL)] 7 - Preparation of BVLD
[0073] Weigh mPEG 10000 -OH 25g (2.5mmol) was placed in a reaction flask, dichloromethane 100mL was added, and the solid was dissolved, then triethylamine 3.75mL (2.5mmol) and p-toluenesulfonyl chloride 4.75g (Ts-Cl, 25mmol) were added, and stirred at room temperature reaction. After the TLC detection reaction is complete, remove the solvent by rotary evaporation, add 150 mL of anhydrous ether to precipitate a solid, add water to dissolve the solid, separate the liquid, separate the water layer, wash twice with 100 mL of anhydrous ether, and then wash the water phase with 150 mL of dichloromethane Extracted twice, collected the organic phase and dried overnight. The solvent was removed by rotary evaporation, the solid was precipitated by adding anhydrous ether, filtered, and dried to obtain mPEG 10000 -OTs 24g, yield 92%.
[0074] mPEG 10000 -OTs 20g (2mmol) was dissolved in 30mL DMF, phthalimide pota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com