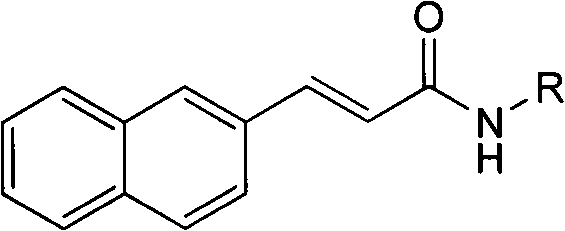
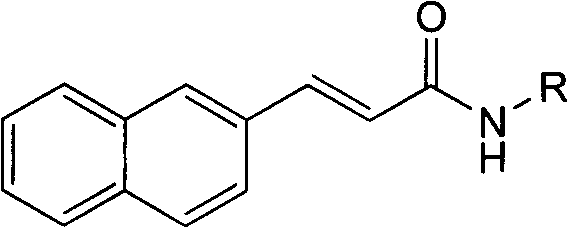
3-(2-naphthyl) acrylamide derivatives as well as preparation method and application thereof
A technology of acrylamide and its derivatives, which is applied in the field of 3-(2-naphthyl)acrylamide derivatives and their preparation and application, and can solve the problems of preventing the growth of malignant tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
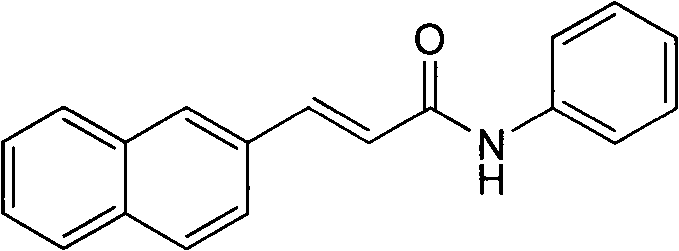
[0020] Example 1: Preparation of 3-(2-naphthyl)acrylanilide (compound 1)
[0021]
[0022] Freshly prepared 3-(2-naphthyl)acryloyl chloride (1 mmol) was dissolved in anhydrous CH 2 Cl 2 (30mL), add NaHCO 3 (0.5g), stirred for 5min, added DMAP (5mg), continued to stir for 5min, added aniline (1mmol) into the system, and refluxed at 40-50°C for 5h. The reaction solution was washed successively with 5% HCl aqueous solution (50mL), 5% NaOH aqueous solution (50mL) and clear water (50mL) for 3 times each, and the organic phase was taken and washed with anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure, and silica gel column chromatography was performed using a mixed solvent of petroleum ether: ethyl acetate = 5:1 as the eluent. The eluate was evaporated under reduced pressure to remove the solvent, and recrystallized in ethyl acetate solution to obtain the target compound as white needle crystals. Yield 75%. Mp: 161-163℃.ESI-MS ([M+H] + ): 27...
Embodiment 2
[0023] Example 2: Preparation of 3-(2-naphthyl)acryloyl-4-isopropylaniline (compound 2)
[0024]
[0025] The preparation method is the same as in Example 1. Substituting 4-isopropylaniline for aniline, the target compound was obtained as pale yellow needle-like crystals. Yield 68%. Mp: 174-175℃.ESI-MS ([M+H] + ): 316.2. 1 H-NMR (400 MHz, DMSO-d 6 , δ ppm): 1.21 (d, 6H), 2.84 (m, 1H), 6.89 (d, 1H), 7.28 (d, 2H), 7.30 (d, 1H), 7.48 (d, 1H), 7.59-7.62 (m, 4H), 7.77 (d, 1H), 7.86 (s, 1H), 8.12 (m, 2H), 9.70 (brs, 1H).
Embodiment 3
[0026] Example 3: Preparation of 3-(2-naphthyl)acryloyl-2-chloroaniline (compound 3)
[0027]
[0028] The preparation method is the same as in Example 1. Substituting 2-chloroaniline for aniline, the target compound was obtained as white needle-like crystals. Yield 70%. Mp: 152-154℃.ESI-MS ([M+H] + ): 308.2. 1 H-NMR (400MHz, DMSO-d 6 , δ ppm): 6.93(d, 1H), 7.17(m, 1H), 7.35(m, 1H), 7.42(d, 1H), 7.44(d, 1H), 7.55(m, 2H), 7.68(d , 1H), 7.73 (s, 1H), 7.85 (d, 1H), 7.99-8.03 (m, 3H), 9.23 (brs, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com