Binding constructs and methods for use thereof
A technology combining structural domains and populations, applied in chemical instruments and methods, antibodies, drug combinations, etc., can solve problems such as short action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
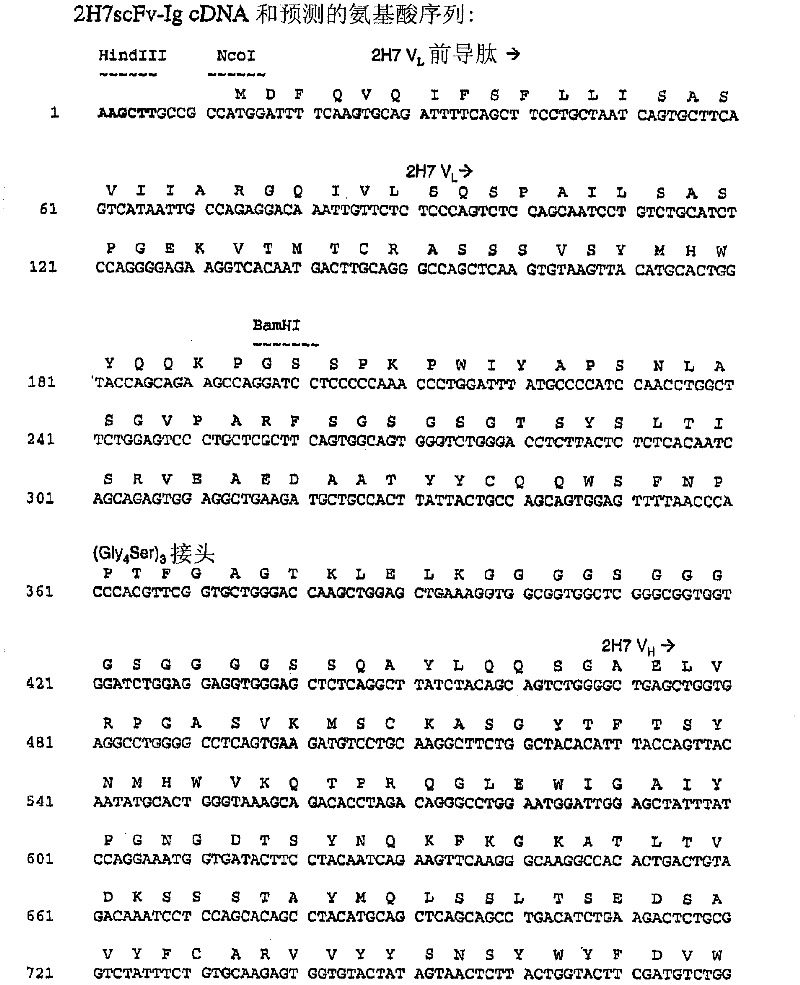
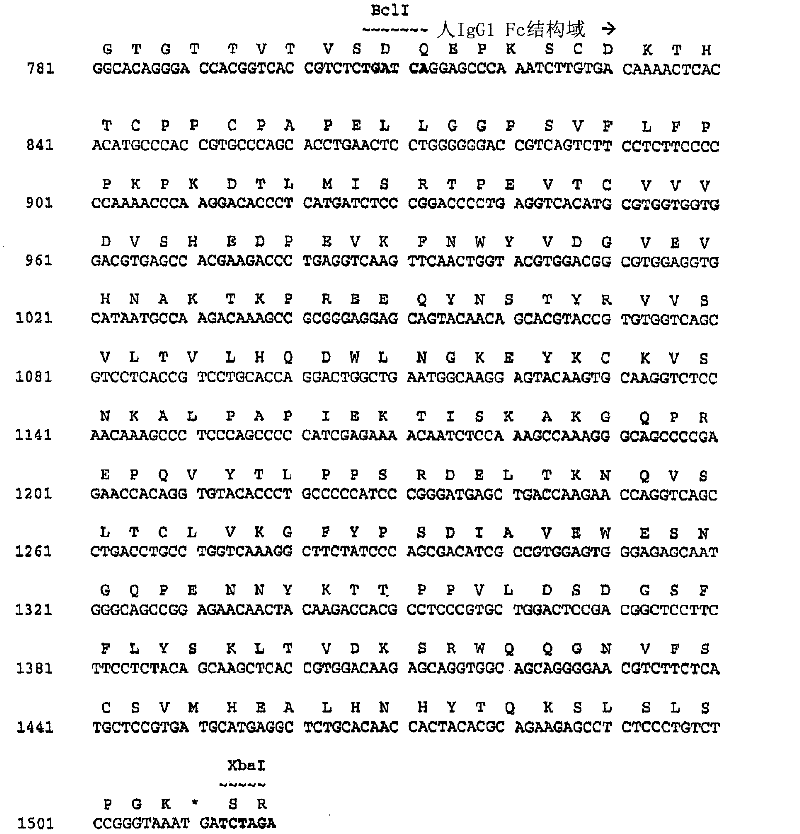
[0551] Cloning of 2H7 variable region and construction and sequencing of 2H7 scFv-Ig
[0552] This example illustrates the cloning of cDNA molecules encoding the heavy and light chain variable regions of monoclonal antibody 2H7. This example also demonstrates the construction, sequencing and expression of 2H7 scFv-Ig.
[0553] Cells expressing the 2H7 monoclonal antibody that specifically binds CD20 were grown in logarithmic phase for several days in RPMI 1640 medium (Invitrogen / Life Technologies, Gaithersburg, MD) supplemented with gluten before harvesting. Aminoamide, pyruvate, DMEM non-essential amino acids, and penicillin-streptomycin. Pellet the cells from the culture medium by centrifugation and use 2x10 7 Cells make RNA. RNA was isolated from 2H7-producing hybridoma cells using the Pharmingen (San Diego, CA) Total RNA Isolation Kit (Cat# 45520K) according to the manufacturer's instructions accompanying the kit. cDNA was prepared by reverse transcription using 1 micr...
Embodiment 2
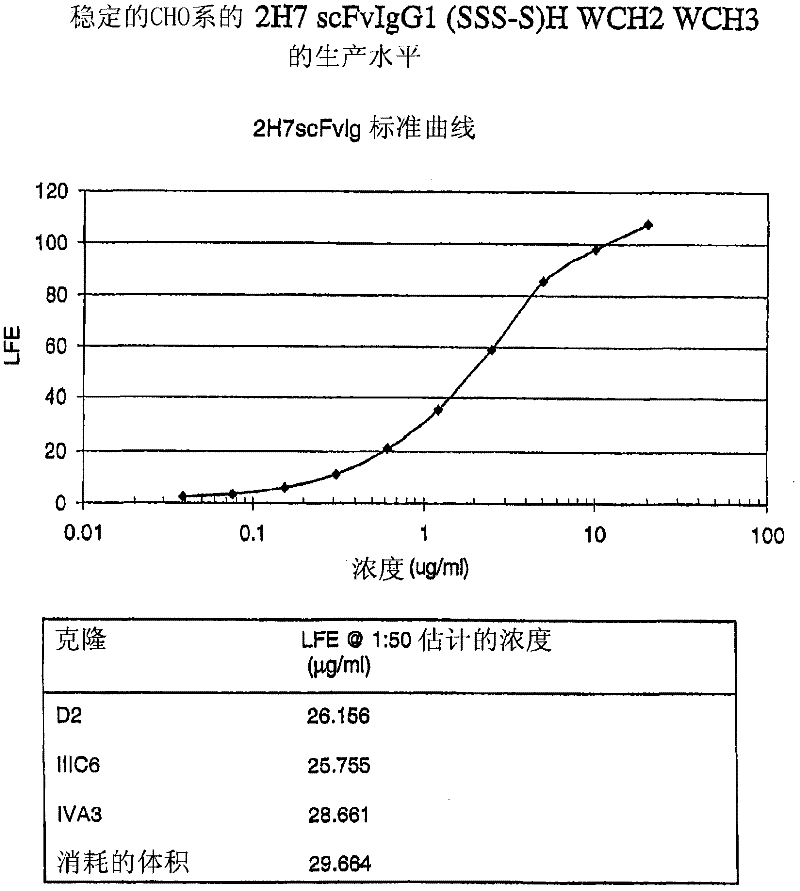
[0559] Expression of 2H7scFv-Ig in Stable CHO Cell Line
[0560] This example illustrates the expression of 2H7scFv-Ig in eukaryotic cell lines and the characterization of expressed 2H7scFv-Ig by SDS-PAGE and functional assays including ADCC and complement fixation.
[0561]The 2H7scFv-Ig HindIII-XbaI (about 1.6kb) fragment with the correct sequence was inserted into the mammalian expression vector pD18, and the DNA of the positive clone was amplified with the QIAGEN plasmid preparation kit (QIAGEN, Valencia, CA). Recombinant plasmid DNA (100 μg) was then linearized in non-essential regions by digestion with AscI, purified by phenol extraction, and resuspended in tissue culture medium Excell 302 (Cat# 14312-79P, JRH Biosciences, Lenexa, KS) middle. The cells used for transfection, CHO DG44 cells, were kept in logarithmic growth, and 10 cells were harvested for each transfection reaction. 7 cells. Linearized DNA was added to CHO cells in a total volume of 0.8 ml for electrop...
Embodiment 3
[0570] Effects of simultaneous ligation of CD20 and CD40 on normal B cell growth, CD95 expression and induction of apoptosis
[0571] This example illustrates the effect of cross-linking of CD20 and CD40 expressed on the cell surface on cell proliferation.
[0572] Dense quiescent B cells were isolated from human tonsils by a Percoll graded gradient and T cells were depleted by E-rosetting. During the last 12 hours of the 4-day experiment, by 3 The uptake of [H]-thymidine, the proliferation of quiescent, densely packed tonsillar B cells was measured. Proliferation was measured in quadruplicate cultures with means and standard deviations as indicated. Mouse anti-human CD20 mAb 1F5 (anti-CD20) alone or cross-linked with anti-mouse kappa mAb 187.1 (anti-CD20XL) was used. CD40 activation was accomplished using soluble human CD154 fused to murine CD8 (CD154) (Hollenbaugh et al., EMBO J 11:4212-21 (1992)) and anti-mouse CD8 monoclonal antibody 53-6 (CD154XL) CD40 cross-linking i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com