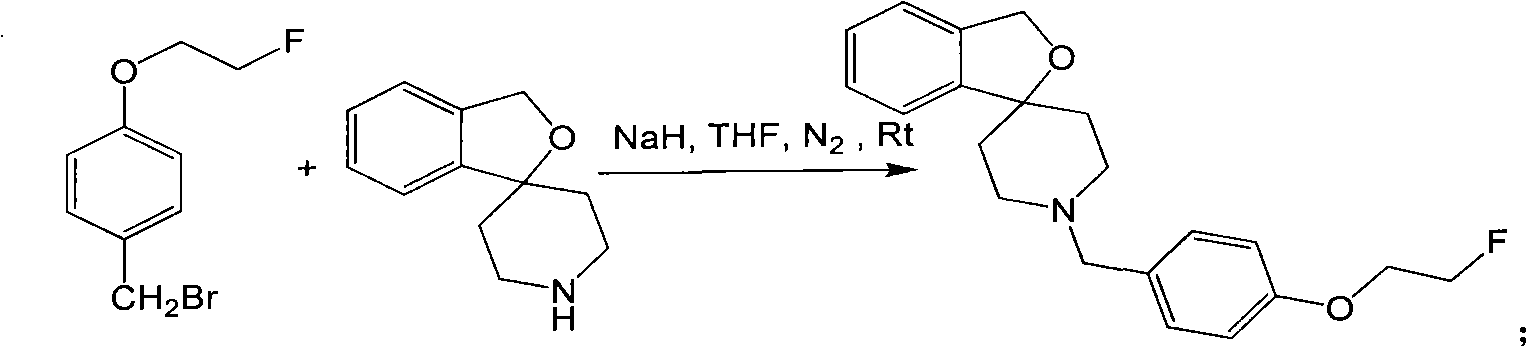Fluorine-18-labeled spiropiperidine sigma-1 receptor compound and its preparation method and use
A technology for spirocyclic piperidine and compound, which is applied in the field of spirocyclic piperidine sigma-1 receptor compounds and preparation, and can solve the problems of low selectivity, short carbon-11 half-life, and unfavorable clinical promotion and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0069] Describe the present invention in detail below by embodiment,
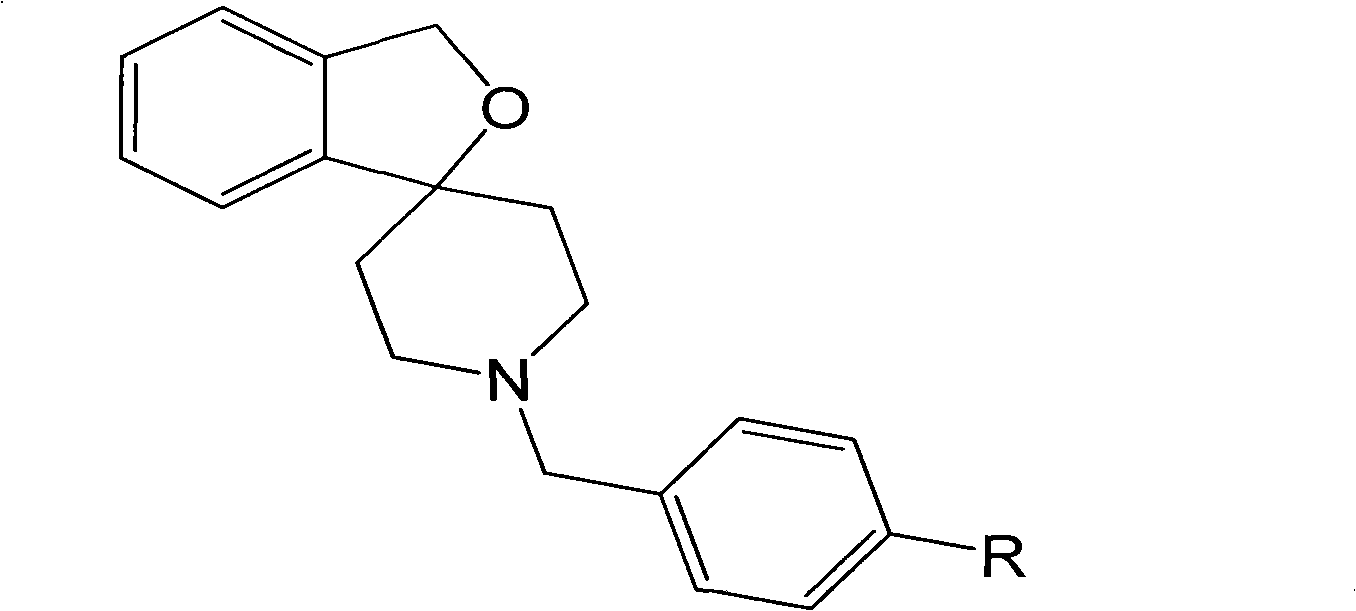
[0070] A fluorine-18 labeled spirocyclic piperidine sigma-1 receptor compound, the general structural formula of which is:
[0071]
[0072] Where: R=OCH 3 , OCH 2 CH 2 F. (OCH 2 CH 2 ) 2 F or (OCH 2 CH 2 ) 3 F;
[0073] Fluorine-18 labeled spirocyclic piperidines σ 1 The preparation method of receptor compound comprises: receptor ligand Spiro-OCH 3 (R=OCH 3 ), synthesis of receptor ligand Spiro-PEG1-F, synthesis of compound intermediate Spiro-OH, synthesis of receptor ligand Spiro-PEG2-F, Spiro-PEG3-F, labeling precursor Spiro-PEGn (n= 1) Preparation of -OTs, labeling precursor Spiro-PEGn(n=3)-OTs and fluorine-18-labeled spirocyclic piperidine compounds, the process steps are as follows:
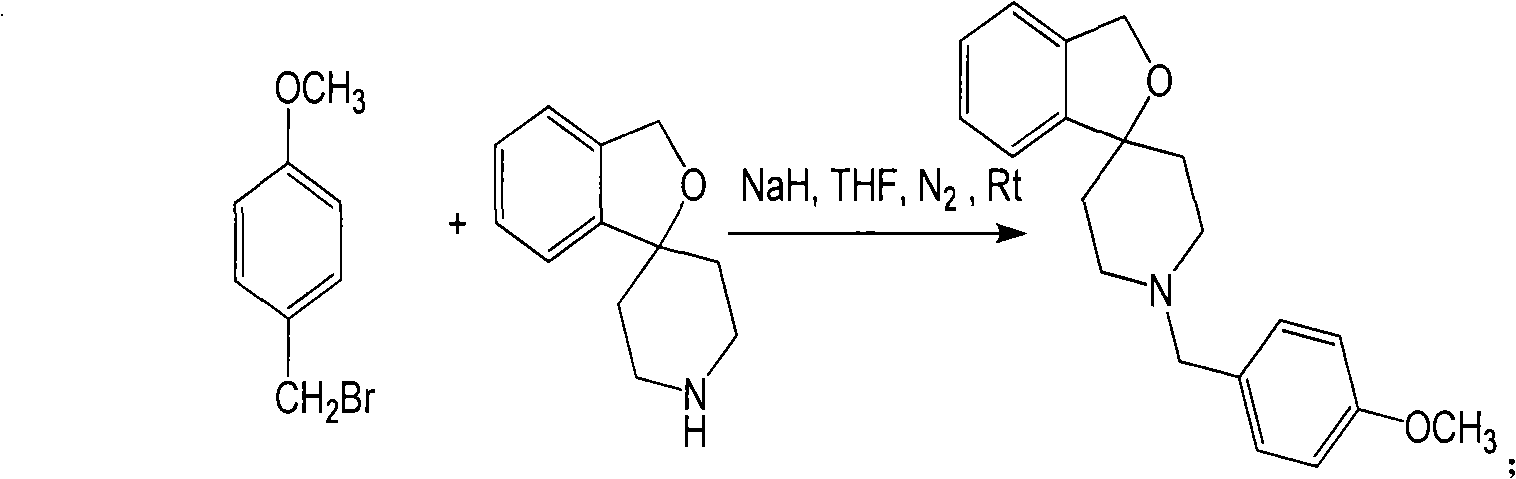
[0074] a. Receptor ligand Spiro-OCH 3 (R=OCH 3 )Synthesis:
[0075] Dissolve 500mg Spiro-piperidine in 20mL acetonitrile, add 2g K 2 CO 3 , 200mg KI, then add 580mg 4-methoxybenzyl bromide, react at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com