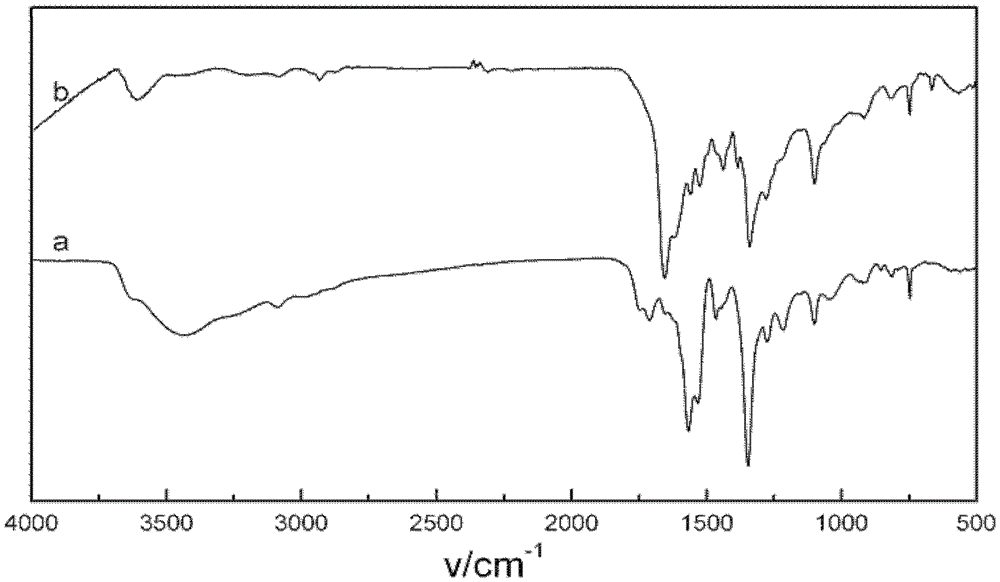
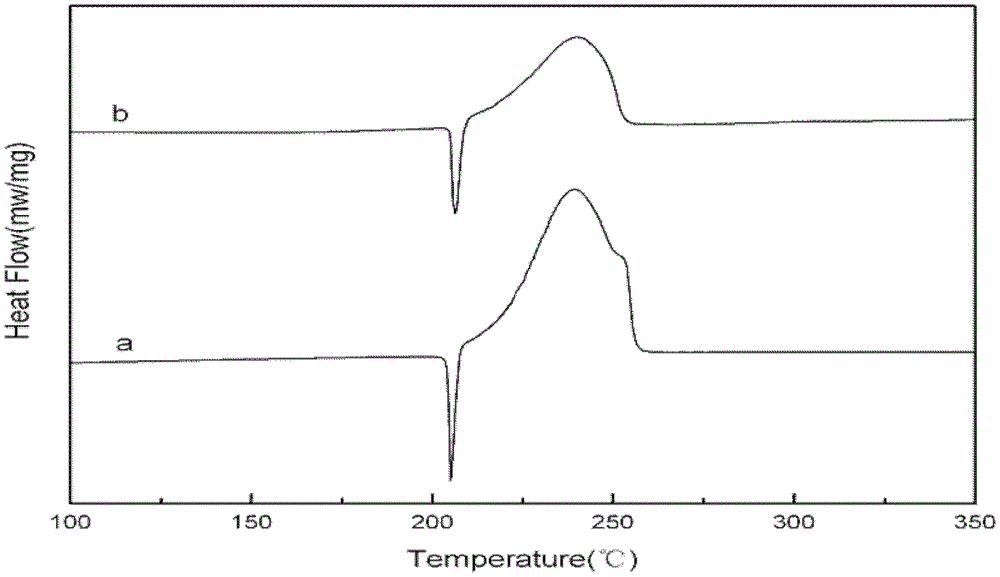
Metal salt complex of p-nitrocalixarene and preparation method thereof
A technology for nitrocalixarene and metal salts, which is applied to nickel organic compounds, zinc organic compounds, cadmium organic compounds, etc., can solve problems such as many by-products, and achieve the effects of less by-products, easy synthesis, and fewer steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In the copper salt complex of p-nitrocalix[8]arene in this embodiment, M is Cu 2+ , a is 8, n is 4, m is 32, j is 16, its general formula is C 56 H 64 N 8 O 40 Cu 4 .
[0028] The preparation method of the copper salt complex of the above-mentioned p-nitrocalix[8] arene can be as follows:
[0029] Step 1: Use p-tert-butylcalix[8]arene, nitric acid and glacial acetic acid as raw materials to prepare p-nitrocalix[8]arene and dissolve it in N,N-dimethylformamide
[0030] Step 1.1: Preparation of p-nitrocalix[8]arene
[0031] Step 1.1.1: Weigh 12g of p-tert-butylcalix[8]arene, and slowly add it into a mixed solution containing 50mL of nitric acid and 115mL of glacial acetic acid, the molar ratio of p-tert-butylcalix[8]arene to nitric acid. is 1: 125, the volume ratio of nitric acid and glacial acetic acid is 1: 2.3, add dropwise while stirring, continue to stir for 5 hours after the dropwise addition, and keep the temperature of the whole process at -2~0°C;
[0032]...
Embodiment 2
[0042]In the metal salt complex of p-nitrocalix[8]arene in this embodiment, M is Cu 2+ , a is 8, n is 3, m is 34, j is 12, the general formula is: C 56 H 58 N 8 O 36 Cu 3 .
[0043] The metal salt complex preparation method of above-mentioned p-nitrocalix[8] arene is as follows:
[0044] In step 2, the Cu(NO 3 ) 2 ·3H 2 03g is dissolved in water to dissolve completely; The copper nitrate solution is added to the N,N-dimethylformamide solution of p-nitrocalixarene, and the molar ratio of p-nitrocalixarene to copper nitrate is 1:3, and in this step Other operations are the same as those in Embodiment 1.
[0045] The other steps were the same as in Example 1, and 4.6 g of the copper salt of p-nitrocalix[8]arene was prepared, and the yield was 80.1%.
Embodiment 3
[0047] In the metal salt complex of p-nitrocalix[8]arene in this embodiment, M is Cu 2+ , a is 8, n is 2, m is 36, j is 8, the general formula is: C 56 H 52 N 8 O 32 Cu 2 .
[0048] above C 56 H 52 N 8 O 32 Cu 2 The preparation method is as follows:
[0049] Weigh out Cu(NO 3 ) 2 ·3H 2 O2g is dissolved in water until completely dissolved; the copper nitrate solution is added to the N,N-dimethylformamide solution of p-nitrocalixarene, so that the molar ratio of p-nitrocalixarene and copper nitrate is 1:2, this step The other operations are the same as in Example 1.
[0050] The other steps were the same as in Example 1, and 4.3 g of the copper salt of p-nitrocalix[8]arene was prepared, and the yield was 77.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com