Method for constructing mammary gland specifically expressed human bactericidal/permeability-increasing protein (hBPI) vector
A protein carrier and construction method technology, applied in the field of bioengineering, can solve problems such as low success rate, low expression level, and long test cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
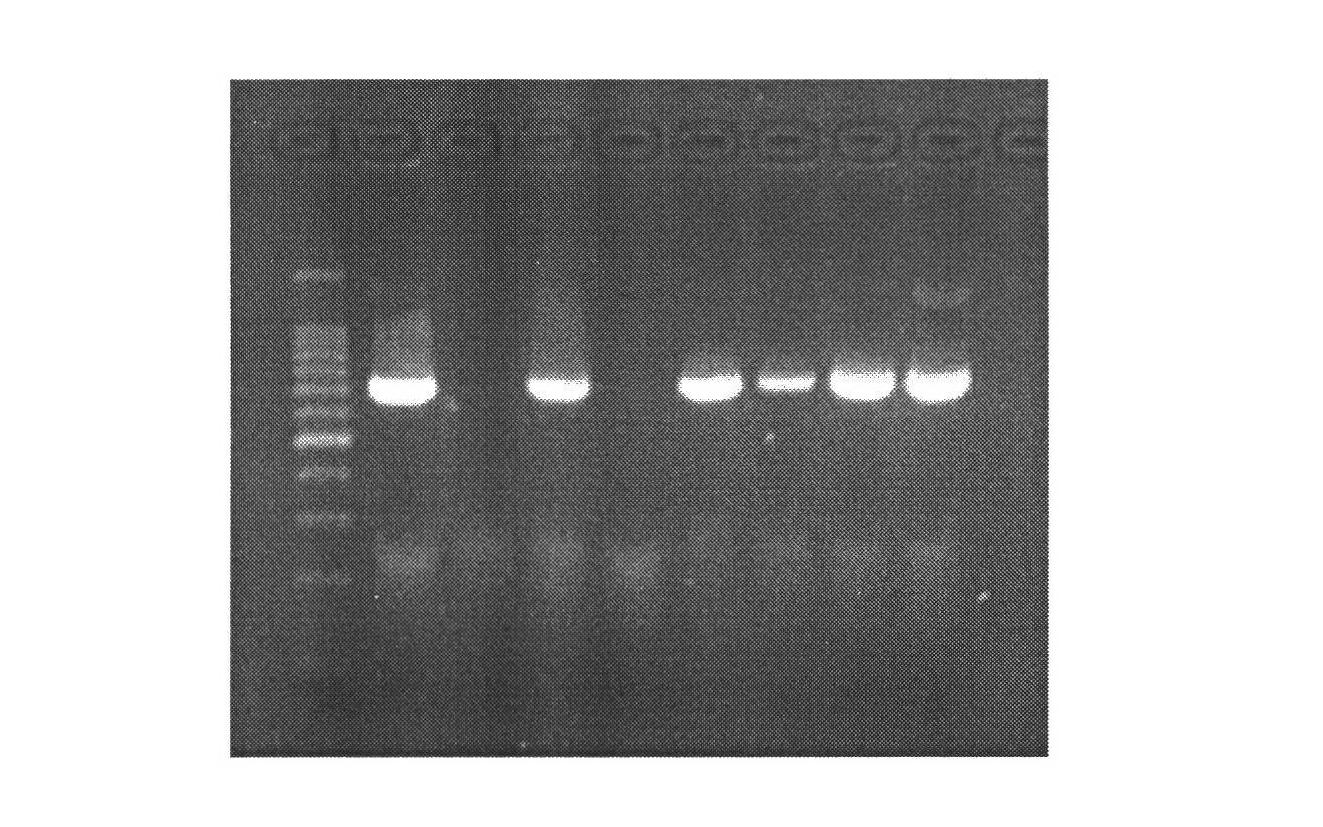
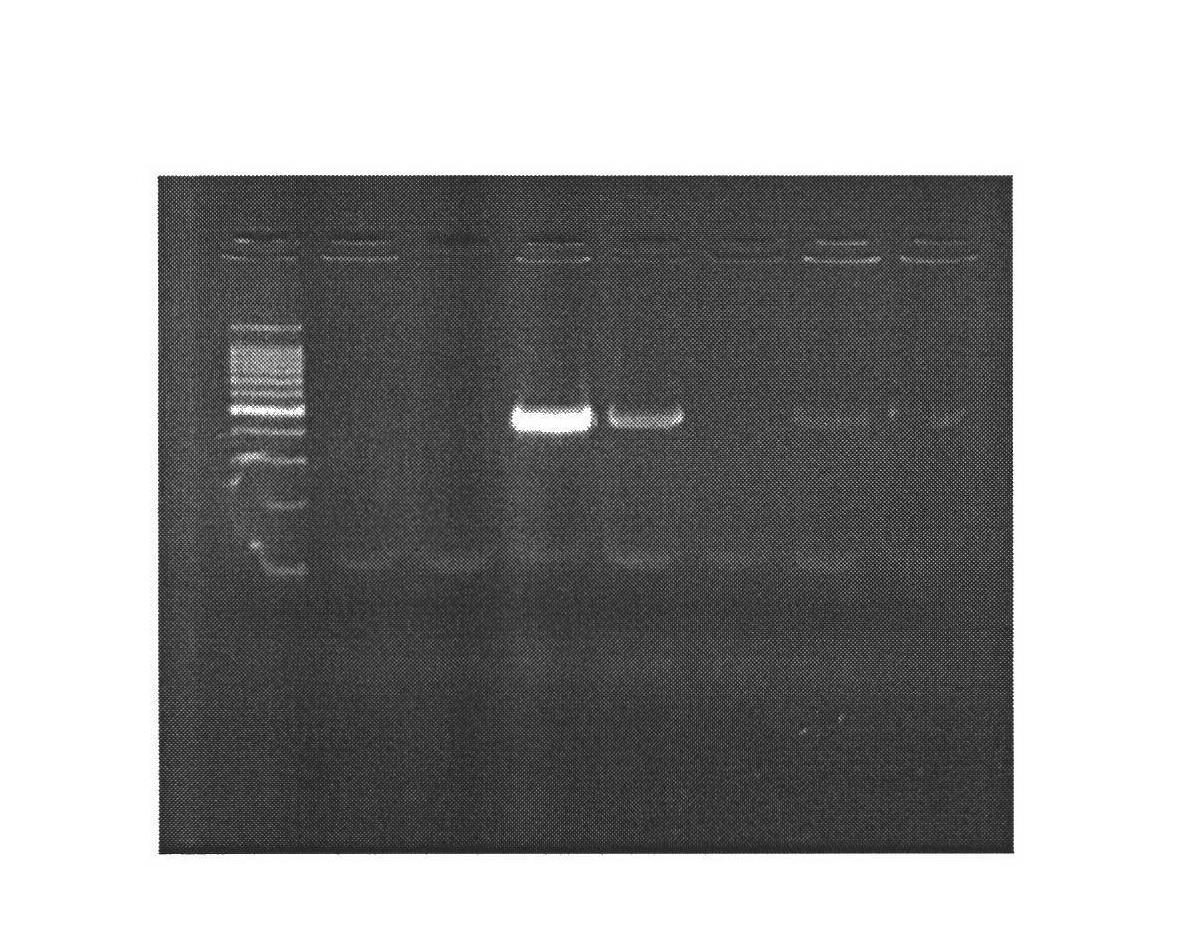
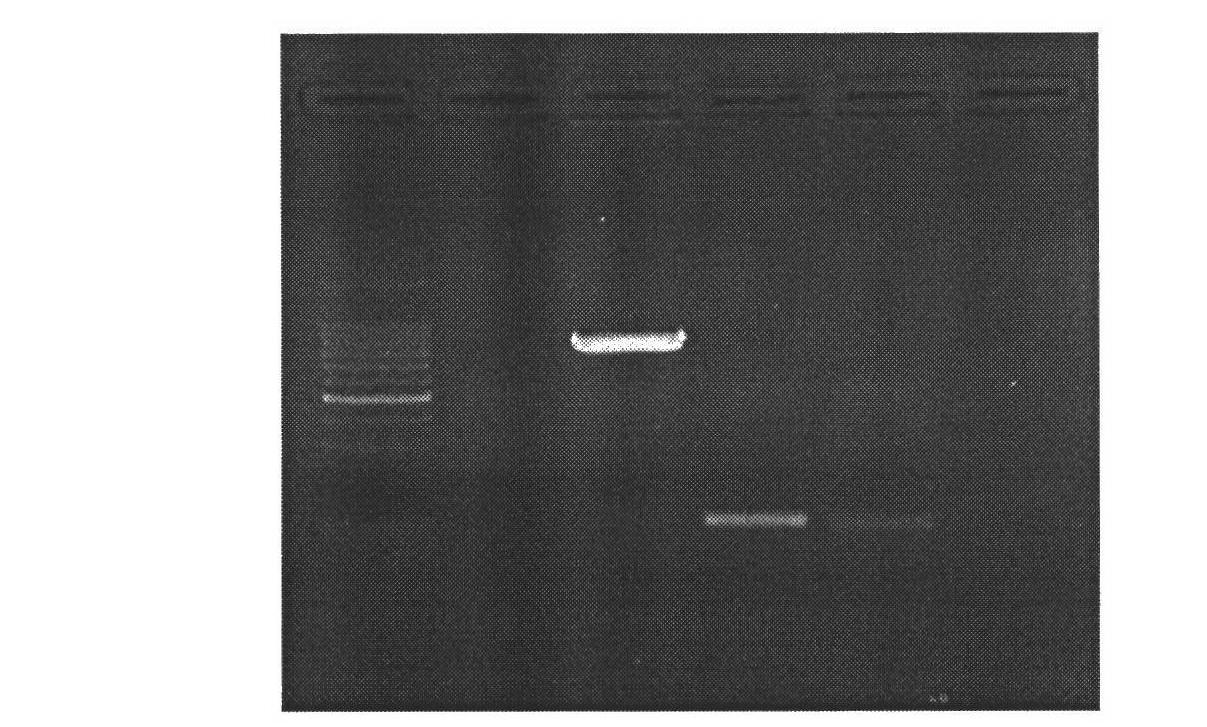
[0028] 1. Mammary gland-specific expression of human bactericidal permeability-enhancing protein (hBPI) vector construction
[0029] (1) According to the human BPI gene sequence (1464bp) (Genebank: NM_001725.2), we designed the sequence as follows: XhoI restriction site + Kozak sequence + bovine β-casein signal peptide + CDS sequence of human BPI + XhoI restriction The site was synthesized by Genscript and cloned into the EcoR V restriction site of the pUC57 vector, named pUC57-hBPI.
[0030] (2) Kit to extract pUC57-hBPI, pBC1-loxp-neo-loxp-pBD plasmids, add plasmids, restriction enzymes Xho I, Buffer, H 2 O constituted a 50 μl reaction system, and digested at 37°C for 4 hours. Gel recovery of the remaining large fragments after digestion of PBC1-PBD, and small fragments after digestion of pUC57-hBPI. The pBC1-loxp-neo-loxp-pBD digestion reaction system is shown in Table 1:
[0031] Table 1
[0032] components
volume
PBC1-PBD
24μl
10×Buffer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com