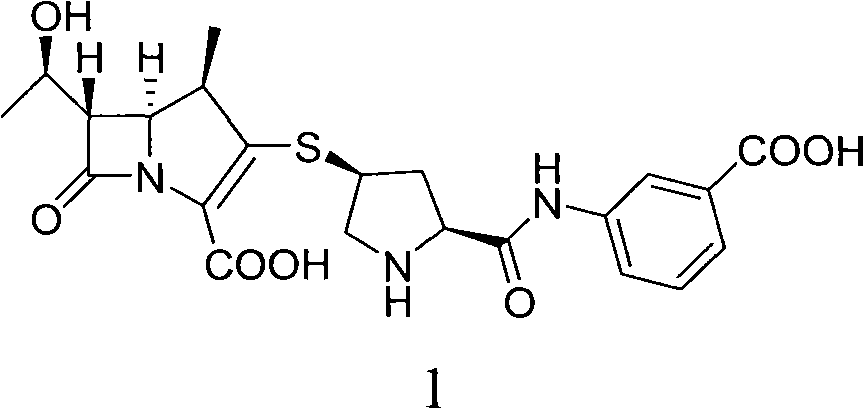
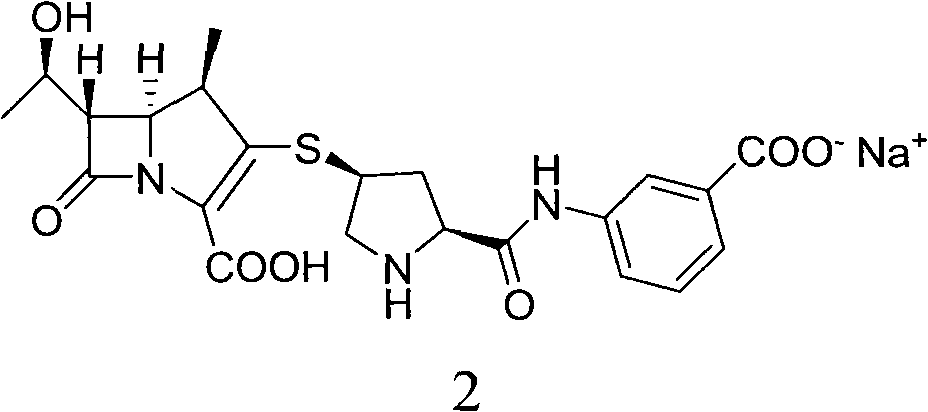
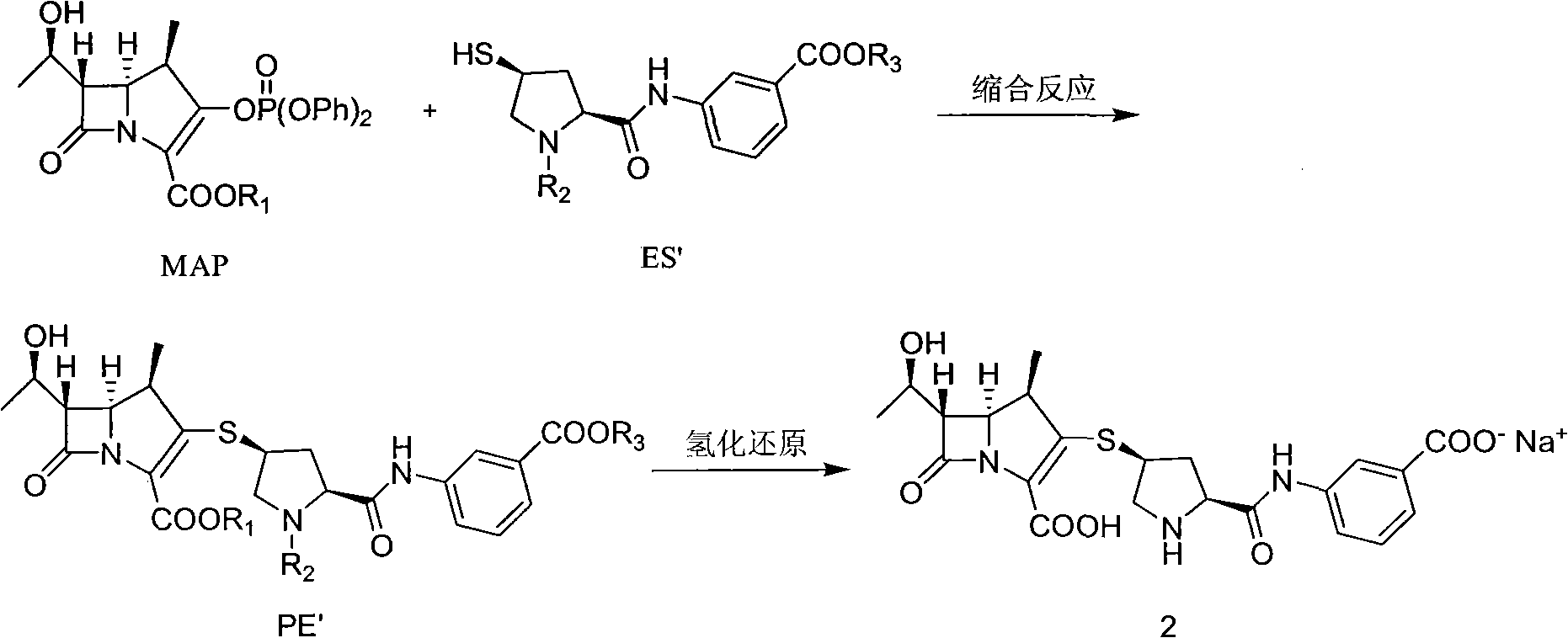
Method for preparing ertapenem intermediate
A technology for ertapenem and ertapenem side chains, which is applied in the field of preparing ertapenem intermediates, can solve problems such as unstable nature and difficult preservation, and achieves the effects of good stability, shortened working hours, and reduced costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 5.0g Ertapenem side chain hydrochloride (R 2 for H), 50mL H 2 O, 2.3 g K 2 CO 3 , 0.03g FeCl 2 .4H 2 O. Air was passed into the reaction at room temperature, followed by HPLC. After the reaction was complete, 2.5 g of concentrated hydrochloric acid (37.5%) was added, and solids were precipitated. Filter, and then recrystallize with 25mL of water to obtain 4.6g ertapenem side chain dimer hydrochloride, off-white solid, which is formula (III) ertapenem side chain dimer hydrochloride, HPLC purity greater than 99 %.
[0065] In a 100mL three-necked bottle, N 2 Under protective conditions, add 1.8 g of the above-mentioned ertapenem side chain dimer hydrochloride, 1.5 mL of tri-n-butylphosphine (n-Bu 3 P), 30mL N,N-dimethylformamide (DMF), dissolved and cooled to -30°C, then slowly added 2.4g diisopropylethylamine (DIPEA) and 0.03g 4-N,N- Dimethylaminopyridine (DMAP), then add 3.0g MAP (R 1 For p-nitrobenzyl), the reaction was stopped after 30min, and the reacti...
Embodiment 2
[0067] Add 5.0g Ertapenem side chain hydrochloride (R 2 for H), Na 2 CO 3 1.85g, 0.03g FeCl 2 .4H 2 O, DMF 50mL was passed into air at room temperature for reaction. After the reaction was followed by HPLC, 3.5 g of concentrated hydrochloric acid (37.5%) was added, and then the reaction solution was added to 1000 mL of acetone, and solids were precipitated. After filtration and recrystallization with 50 ml of water, 4.1 g of a light yellow solid was obtained, which was ertapenem side chain dimer hydrochloride of formula (III), and the HPLC purity was greater than 99%.
[0068] In a 100mL three-necked bottle, N 2 Under protective conditions, 1.98 g of the above-mentioned ertapenem side chain dimer hydrochloride, 1.5 mL of tri-n-butylphosphine (n-Bu 3 P), 15mL N, N-dimethylformamide (DMF), 3.0gMAP (R 1 nitrobenzyl), dissolved and cooled to -40°C, then slowly added dropwise 2.1g tetramethylguanidine (TMG), stopped the reaction after 60min, and added the reaction solution d...
Embodiment 3
[0070] Add 5.0g Ertapenem side chain hydrochloride (R 2 for H), Na 2 CO 3 1.85g, 0.03g FeCl 2 .4H 2 O, DMF 50mL was passed into air at 0~5℃ to react. After the reaction was followed by HPLC, 3.0 g of concentrated hydrochloric acid (37.5%) was added after the reaction, and then the reaction solution was added to 1000 mL of acetone, and solids were precipitated. Filter, then recrystallize with 25 mL of water and 1.25 g of acetic acid to obtain 4.3 g of a light yellow solid, which is the side chain dimer hydrochloride of ertapenem of formula (III), and the HPLC purity is greater than 99%.
[0071] In a 100mL three-necked bottle, N 2 Add 4.1 g of the above-mentioned ertapenem side chain dimer hydrochloride, 4.5 mL of tri-n-butylphosphine (n-Bu 3 P), 90mL N, N-dimethylformamide (DMF), then add 9.0g MAP (R 1 For p-nitrobenzyl), dissolve and cool to 0°C, then slowly add 6.3g tetramethylguanidine (TMG) and 0.09g 4-N, N-dimethylaminopyridine (DMAP) dropwise, stop the reaction af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com