Preparation method of type I clopidogrel hydrogen sulfate
A technology of clopidogrel bisulfate and clopidogrel base, which is applied in the field of preparation of type I clopidogrel bisulfate, which can solve the problems of human poisoning, ether flammability, unsafety, etc., and achieves little harm to human body and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
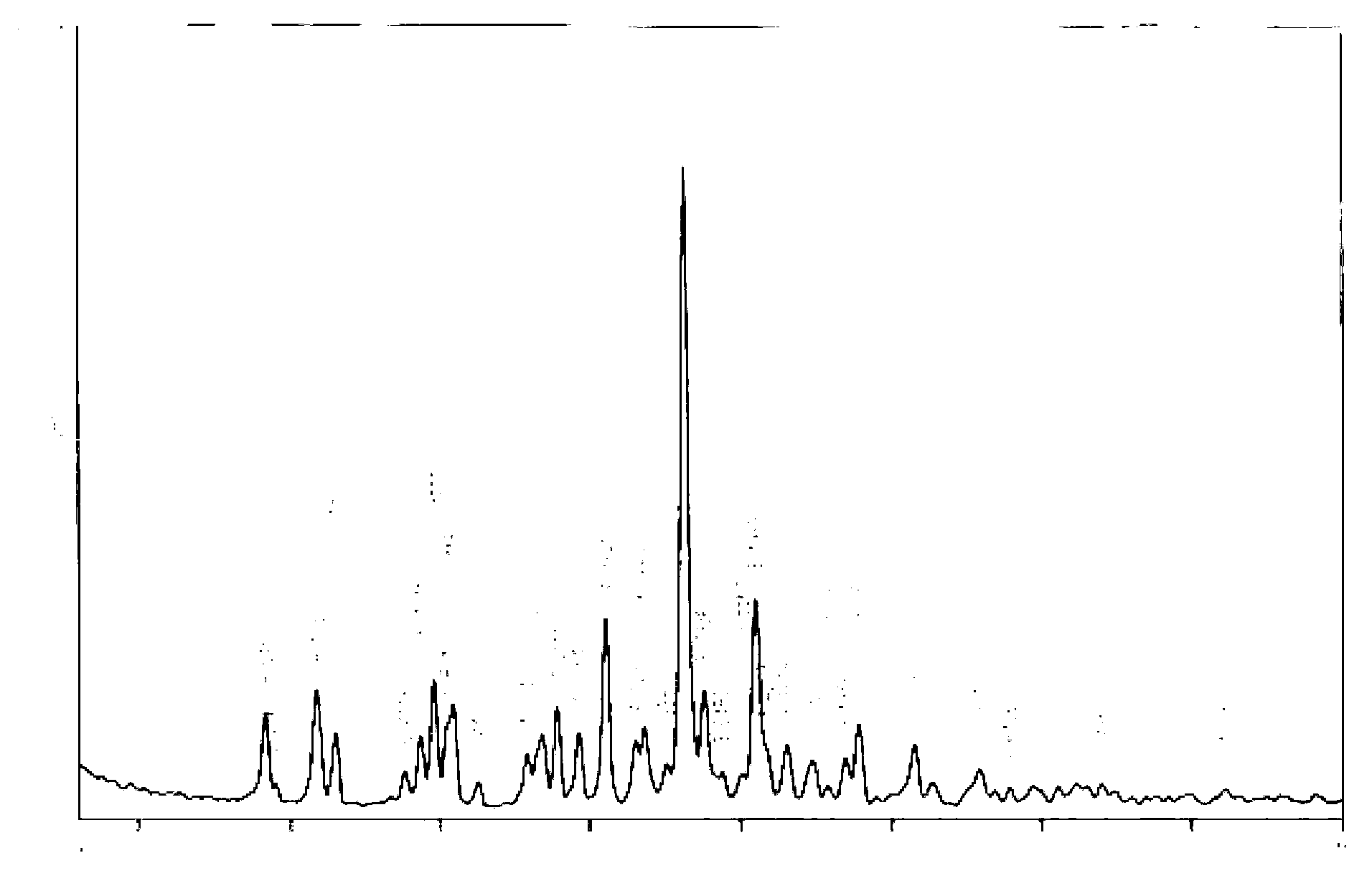
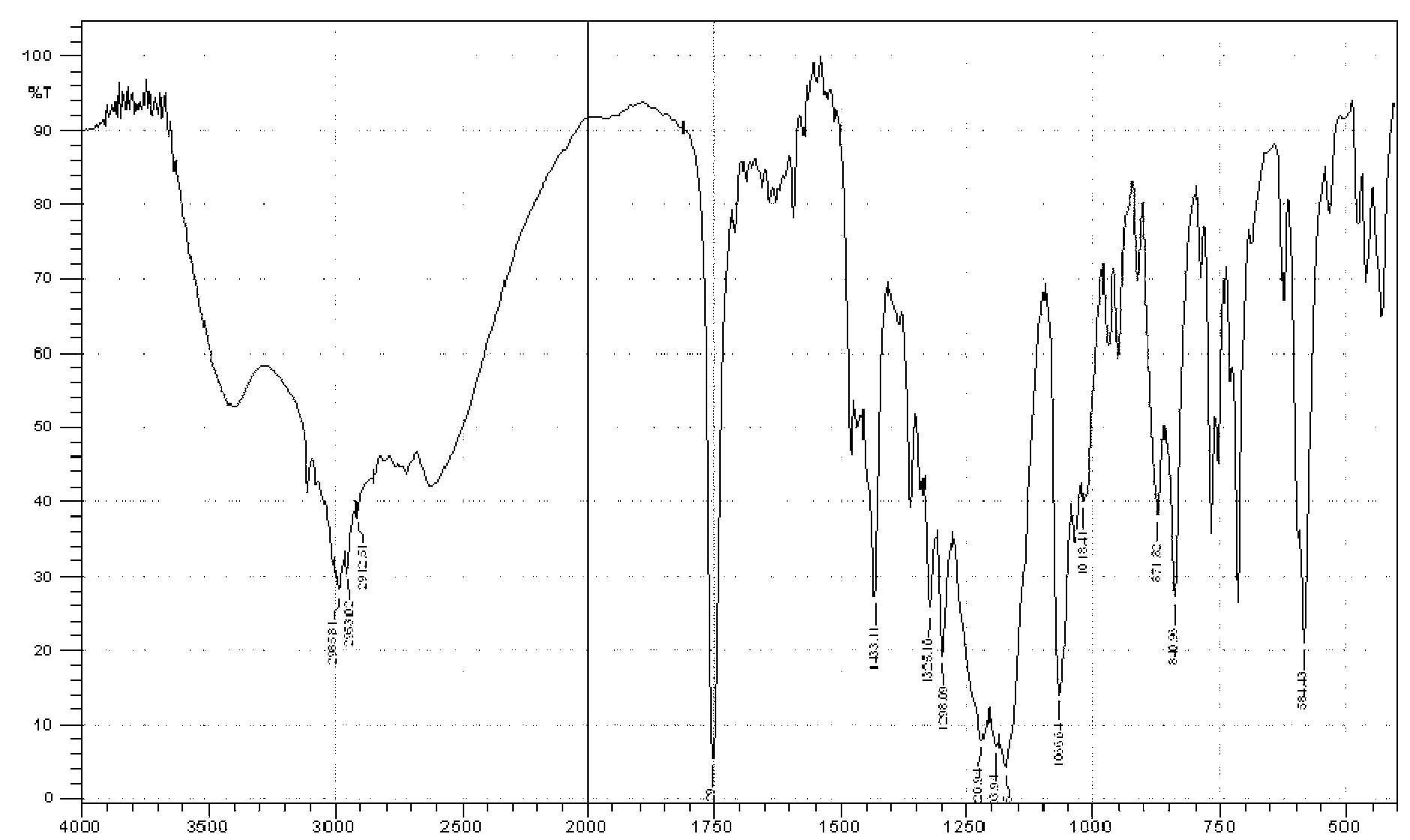
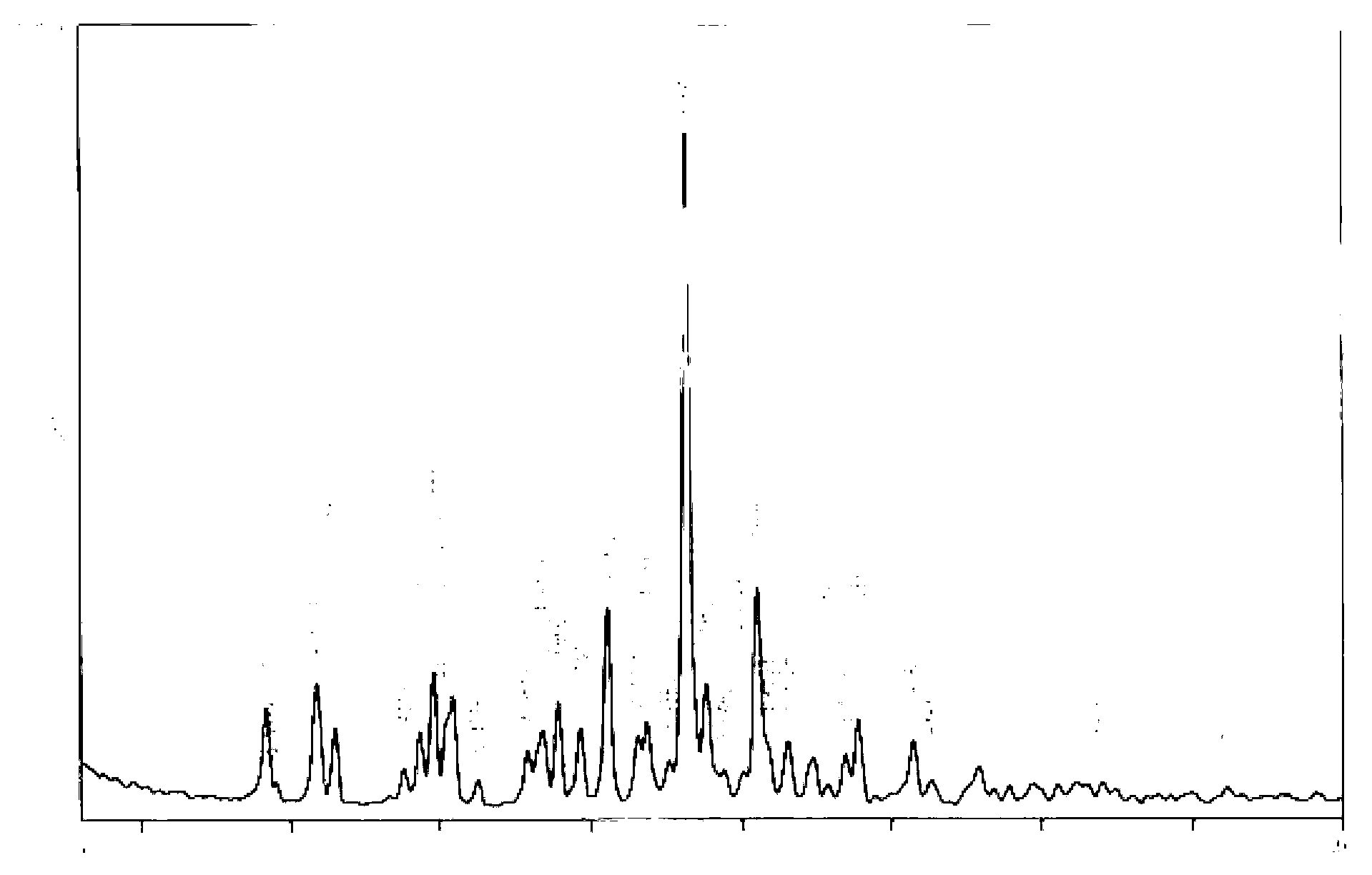
Image
Examples
Embodiment 1
[0032] Put 11.00 g of clopidogrel hydrogen sulfate into a 250 mL round bottom flask, then add 40.0 mL of dichloromethane and 40.0 mL of purified water, control the temperature at 8°C, and adjust the pH to 7 with sodium bicarbonate. The reaction was stirred for 2 hours at 8°C. After the reaction was completed, the layers were separated, the aqueous phase was extracted with 10 mL of dichloromethane, the organic phases were combined, and the organic phase was washed with 10 mL of purified water. Add 1.50 g of anhydrous magnesium sulfate to the organic phase, stir and dry for 5 h. After drying, filter and evaporate the solvent at 40±3°C and -0.08~-0.1MPa to obtain 7.90g of clopidogrel base as an oil. After the distillation is complete, add 135mL of isopropyl ether and 15mL of isopropanol, and cool down to 6°C. After the clopidogrel base is completely dissolved, slowly add 2.65g of concentrated sulfuric acid at this temperature. After the dropwise addition, stir the reaction for 1...
Embodiment 2
[0038]Take 27.50 g of clopidogrel hydrogen sulfate and put it in a 500 mL round bottom flask, then add 100.0 mL of dichloromethane and 100.0 mL of purified water, cool down to 8°C, and adjust the pH to 9 with sodium carbonate. The reaction was stirred for 2 hours at 8°C. After the reaction was completed, the liquids were separated, the aqueous phase was extracted with 30 mL of dichloromethane, the organic phases were combined, and the organic phases were washed with 30 mL of purified water. Add 3.80g of anhydrous magnesium sulfate to the organic phase, stir and dry for 5h. After drying, filter and distill off the solvent at 40±3°C, -0.08~-0.1MPa to obtain 20.40 g of clopidogrel base as an oil. After the distillation is complete, add 165mL of isopropyl ether and 165mL of isopropanol, and cool down to 6°C. After the clopidogrel is completely dissolved, slowly add 6.69g of concentrated sulfuric acid at this temperature. After the dropwise addition, stir the reaction for 16 hours...
Embodiment 3
[0044] Put 46.2 g of clopidogrel hydrogen sulfate into a 1000 mL round bottom flask, then add 168.0 mL of dichloromethane and 168.0 mL of purified water, cool down to 8°C, and adjust the pH to 8 with sodium bicarbonate. The reaction was stirred for 2.5 hours at 8°C. After the reaction was completed, the liquids were separated, the aqueous phase was extracted with 50 mL of dichloromethane, the organic phases were combined, and the organic phases were washed with 50 mL of purified water. Add 6.00g of anhydrous magnesium sulfate to the organic phase, stir and dry for 5h. After drying, filter and evaporate the solvent at 40±3°C and -0.08~-0.1MPa to obtain 32.50g of oily clopidogrel base. After the distillation is complete, add 63.0mL of isopropyl ether and 567.0mL of isopropanol, and cool down to 6°C. After the clopidogrel is completely dissolved, slowly add 9.90g of concentrated sulfuric acid at this temperature. After the dropwise addition, stir for 16 hours to complete the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com