Application of complement factor H (CFH) to genetic expression products of methamphetamine (METH) addiction patient
A technology of methamphetamine and factor, applied in the field of protein detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
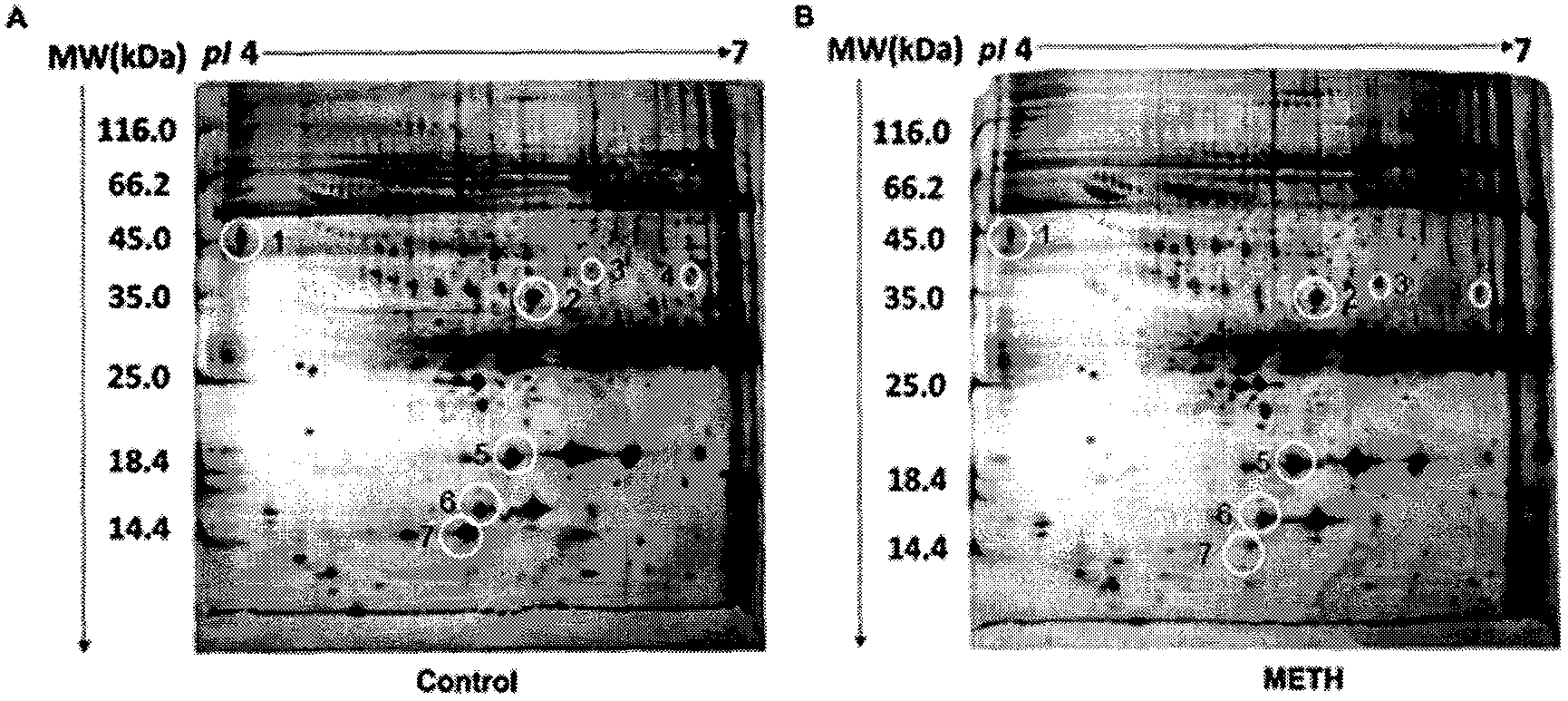
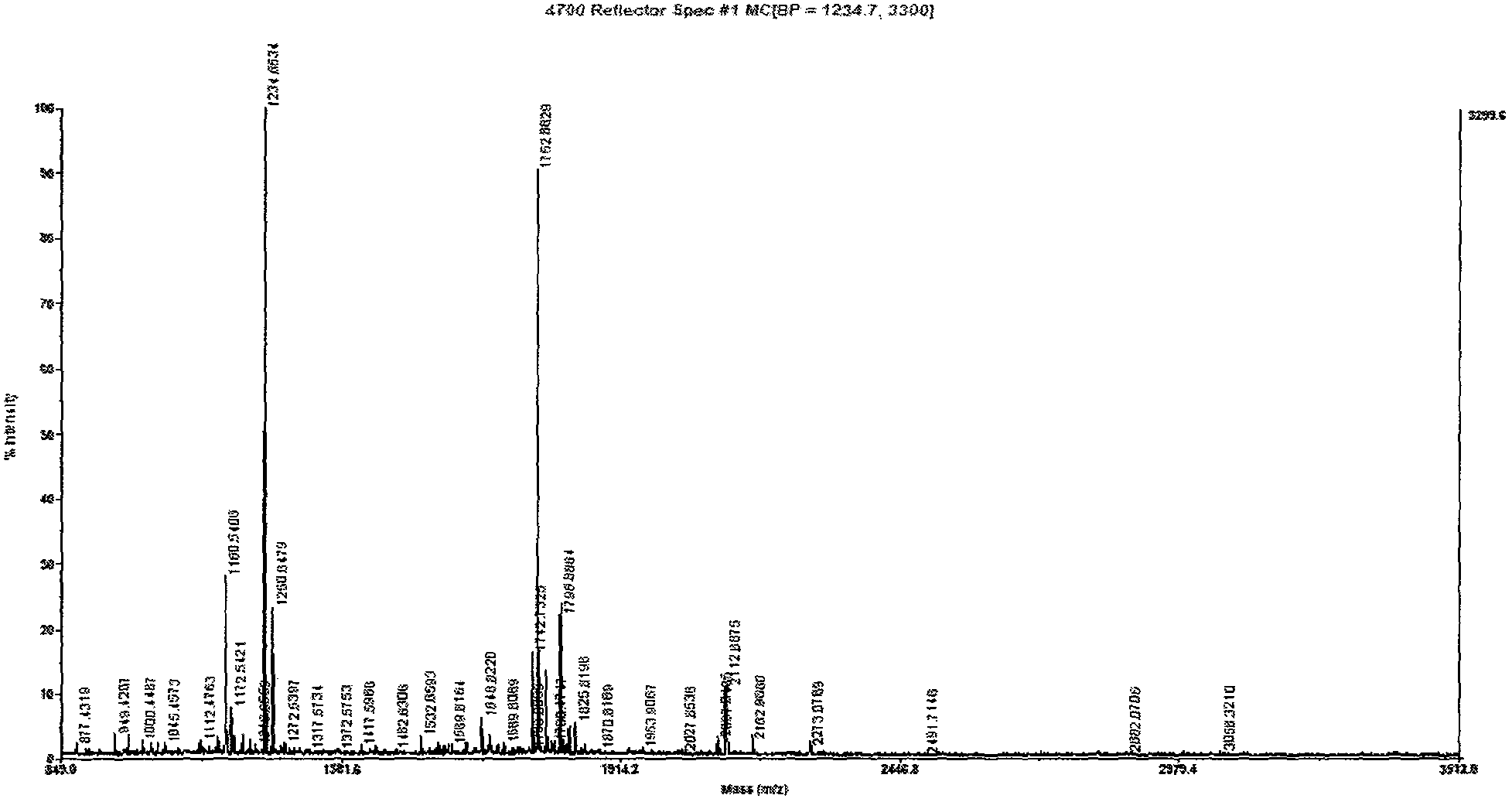
[0073] Example 1 Preparation of human serum samples and isolation of CFH
[0074] 1. Subject population
[0075] METH-addicted patients were recruited from Shenzhen Compulsory Isolation Drug Rehabilitation Center with the assistance of China Institute of Drug Dependence, Peking University; normal controls (healthy volunteers) were recruited from Peking University Third Hospital. After all subjects signed the informed consent, they were evaluated and diagnosed by addiction-related professionals. The selection of patients with METH addiction is based on the "Measures for the Identification of Drug Addiction" of the People's Republic of China (Decree No. 115 of the Ministry of Public Security), "Guiding Principles for the Diagnosis and Treatment of Amphetamine Dependence" and relevant international standards, the tenth edition of the International Classification of Diseases (International Classification of Diseases, ICD-10) and amphetamines in the fourth edition of the Diagnos...
Embodiment 2
[0105] Example 2 Conditioned place preference (CPP) experiment in rats and the acquisition of rat serum and related brain tissue
[0106] In order to confirm the results of serum proteomics in patients with METH addiction, we prepared a rat model of METH addiction, and after the successful verification of the model by CPP experiment, we obtained the model's serum and addiction-related brain tissue for verification of differentially expressed proteins.
[0107] Male SD rats, 30, were then divided into a control group (group C) and a model group (group M), 15 in each group. "Handle" the rat every day (catch the rat with the glove used for drug administration and stroke its abdomen) to adapt to the subsequent intraperitoneal drug administration, and start the formal experiment after 3 days of adaptation. The formal experiment is divided into three parts: pre-conditioning test, conditioning and post-conditioning test (see Figure 17 A). The main experimental methods are as fol...
Embodiment 3
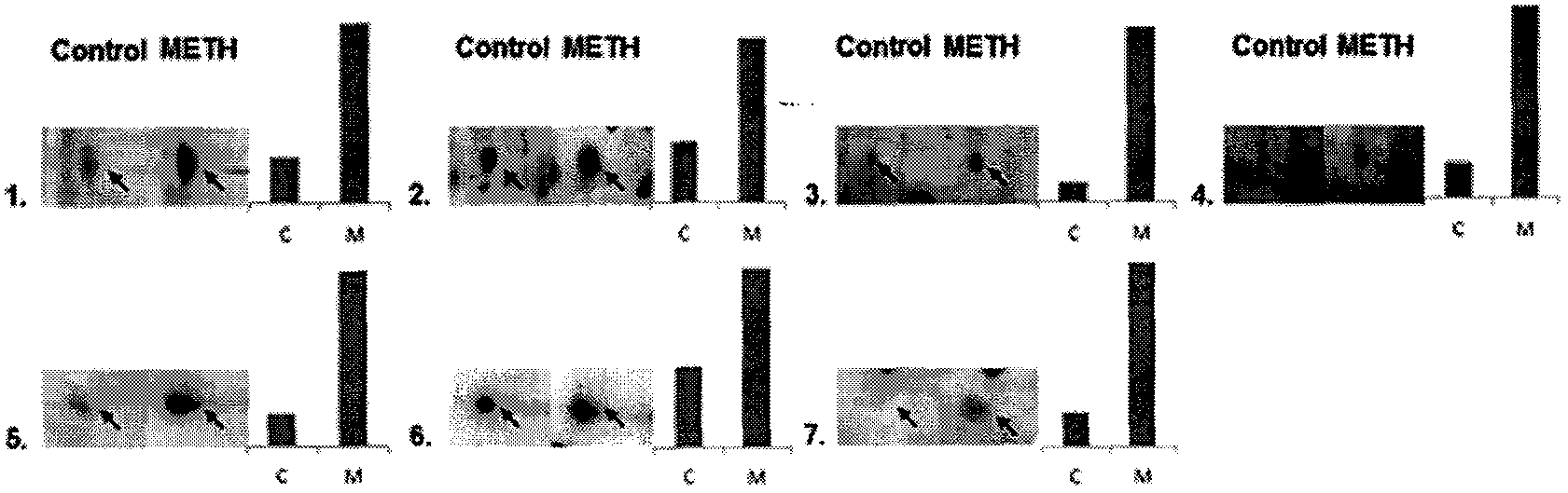
[0116] Example 3 Western blot analysis
[0117] In order to verify the results obtained by 2-DE and mass spectrometry, and to confirm the differential expression of CFH in the serum of addicted people and rats and normal control serum, the following Western blot analysis experiments were performed:
[0118] ① Western blot analysis of patient serum
[0119] Serum samples were diluted 30-fold with Tris-HCl (0.01M, pH 7.4) buffer and loaded. SDS-PAGE used 7.5% polyacrylamide gel as the separating gel. 5% skimmed milk was used for blocking, the primary antibody was CFH monoclonal antibody (Santa Cruz, U.S.A), and the dilution ratio was 1:500. The secondary antibody was HRP-labeled IgG (Proteintech Group, U.S.A), with a dilution ratio of 1:2500. To ensure consistent protein loading, an internal reference was added. GAPDH was used as an internal reference, the primary antibody was GAPDH polyclonal antibody (Millipore, U.S.A), and the dilution ratio was 1:1000; the secondary ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com