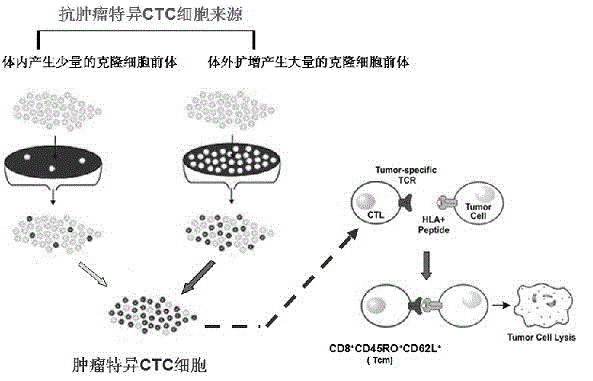
In vitro amplification method of self-specific T cell, prepared T cell system, pharmaceutical use of cell system and component monitoring method of cell system
An in vitro amplification and specific technology, applied in the fields of drug combination, animal cells, individual particle analysis, etc., can solve the problems that hinder the standardization and implementation of new technologies, insufficient cell expansion, lack of quality control monitoring and evaluation methods, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
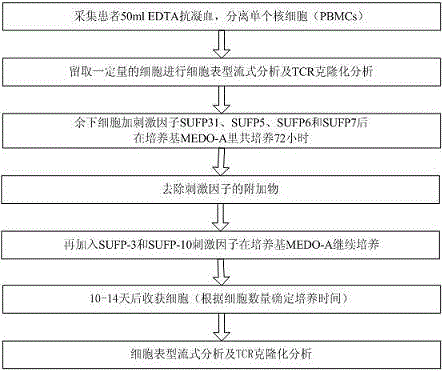
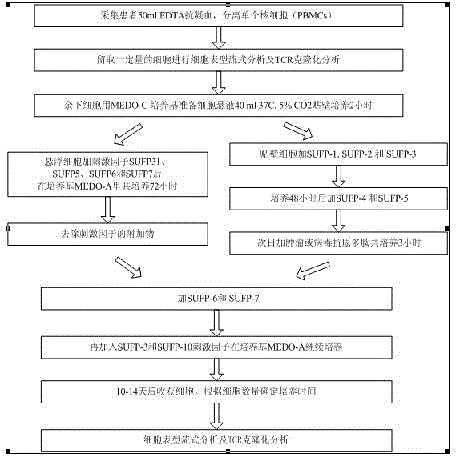
[0063] see figure 2In the example shown, firstly, 50ml of EDTA anticoagulated blood is collected from the patient, and then the peripheral blood mononuclear cells (PBMCs) are separated by the density gradient method, and a certain amount of peripheral blood mononuclear cells (PBMCs) is retained according to the need for cell phenotype flow. Formula analysis and TCR polymorphism analysis, and then the remaining peripheral blood mononuclear cells were prepared with medium MEDO-A cell suspension 40ml, and then after adding the stimulating factors SUPF-31 and SUPF-5, SUPF-6, SUPF-7 Co-cultivate in the medium MEDO-A for 48-72 hours, then add SUPF-3 and SUPF-10 stimulating factors, continue to culture in the medium MEDO-A, and harvest the cells after 10-14 days (the specific culture time can be determined according to the number of cells) ), followed by cell phenotype flow analysis and TCR cloning analysis.
[0064] In order to better explain the present invention and understand i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com