Direct-methanol fuel cell anode catalyst and preparation method
A methanol fuel cell and catalyst technology, applied in battery electrodes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as catalyst loss, and achieve the effects of convenient operation, excellent catalytic activity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0020] (1) 0.200 mmol of six-membered melon ring and 0.067 mmol of H 2 PtCl 6 ·6H 2 O was added to 15 mL deionized water, and stirred for one hour to obtain a uniformly mixed light yellow turbid liquid A;
[0021] (2) Add 0.30 g potassium bromide and 0.24 g ascorbic acid to the cloudy liquid A obtained in step (1) to obtain mixture B;
[0022] (3) Add dropwise 1 mol / L potassium hydroxide aqueous solution to the mixture B obtained in step (2), adjust the pH value to be equal to 9, and obtain mixture C;
[0023] (4) Put the mixture C obtained in step (3) in an oil bath preheated to 120°C for 6 hours, and cool it down to room temperature naturally;
[0024] (5) The product obtained in step (4) was centrifuged (7900 rpm), washed twice with 30 mL of ethanol, and dried at 50°C for 10 hours to obtain the anode catalyst for direct methanol fuel cell of the present invention.
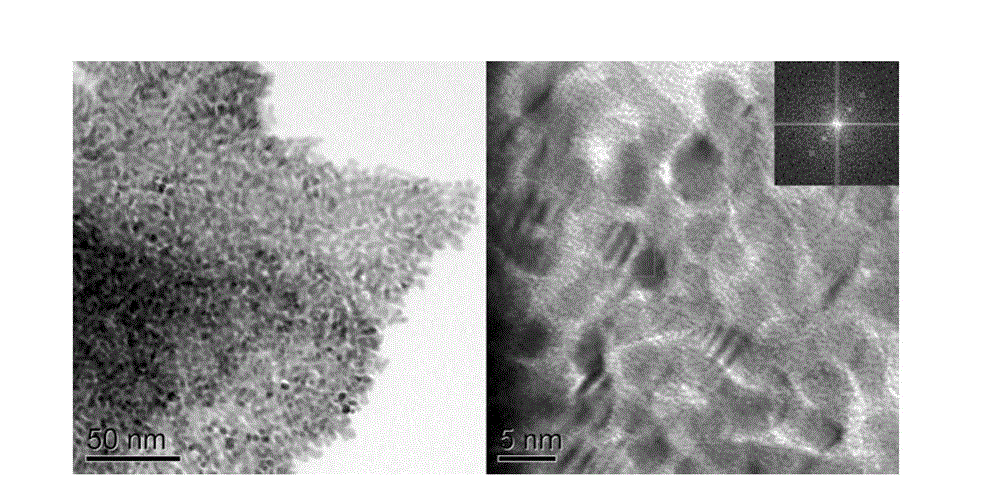
[0025] figure 1 The transmission electron micrograph of the catalyst that provides for implementation ca...
Embodiment example 2
[0030] (1) 0.200 mmol of six-membered melon ring and 0.067 mmol of H 2 PtCl 6 ·6H 2 O was added to 15 mL deionized water, and stirred for one hour to obtain a uniformly mixed light yellow turbid liquid A;
[0031] (2) Add 0.30 g of potassium bromide and 15 mL of ethylene glycol to the cloudy liquid A obtained in step (1) to obtain mixture B;
[0032] (3) Put the mixture B obtained in step (2) in an oil bath preheated to 120°C for 6 hours, and let it cool down to room temperature naturally;
[0033] (4) The product obtained in step (3) was centrifuged (7900 rpm), washed twice with 30 mL of ethanol, and dried at 50°C for 10 hours to obtain the anode catalyst for direct methanol fuel cell of the present invention.
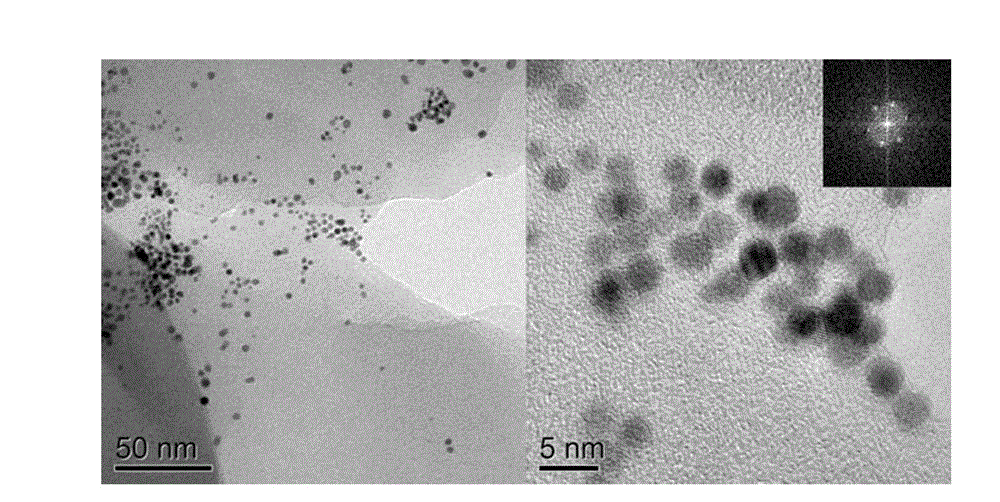
[0034] figure 2 The transmission electron micrograph of the catalyst that provides for implementation case 2, from figure 2 It can be seen that the synthesized catalysts are approximately spherical, the particle size is about 5nm and the distribution is uniform...
Embodiment example 3
[0039] (1) 0.200 mmol of six-membered melon ring and 0.067 mmol of H 2 PtCl 6 ·6H 2 O was added to 15 mL deionized water, and stirred for one hour to obtain a uniformly mixed light yellow turbid liquid A;
[0040] (2) Add 0.30 g of potassium bromide, 0.24 g of ascorbic acid and 15 mL of ethylene glycol to the turbid liquid A obtained in step (1) to obtain mixture B;
[0041] (3) Put the mixture B obtained in step (2) in an oil bath preheated to 120°C for 6 hours, and let it cool down to room temperature naturally;
[0042] (4) The product obtained in step (3) was centrifuged (7900 rpm), washed twice with 30 mL of ethanol, and dried at 50°C for 10 hours to obtain the anode catalyst for direct methanol fuel cell of the present invention.
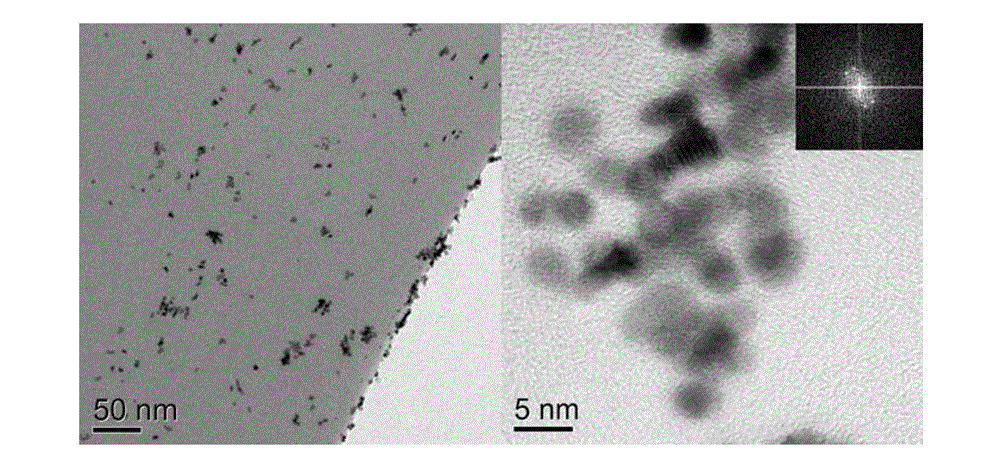
[0043] image 3 The transmission electron micrograph of the catalyst that provides for implementation case 2, from image 3 It can be seen that the synthesized catalysts are all multi-legged, the particle size is about 5nm and the distrib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com