Low BET ball-type manganic manganous oxide and its preparation method
A manganese tetroxide, spherical technology, used in manganese oxide/manganese hydroxide, chemical/physical/physical-chemical fixed reactors, thin material processing, etc. Lithium) materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
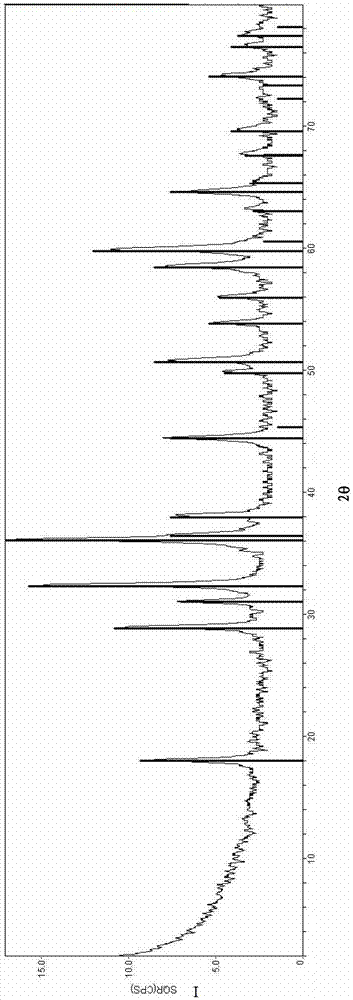
Examples
Embodiment 1
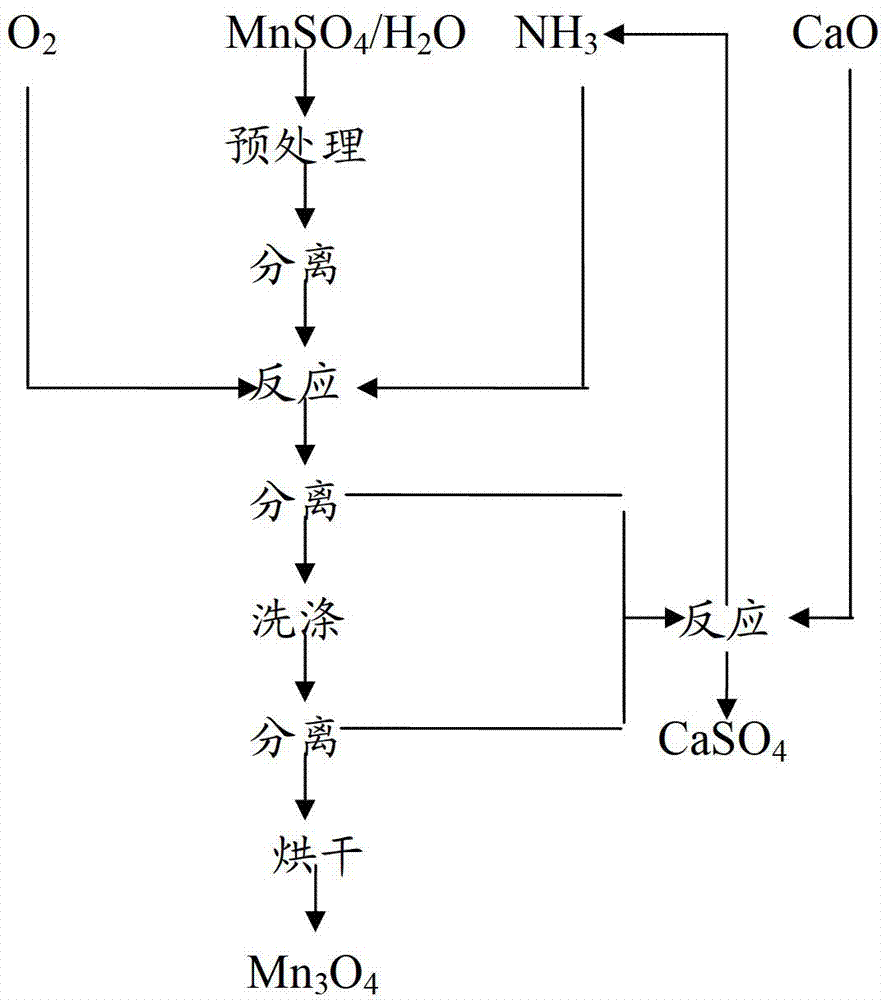
[0086] (1) Pretreatment process of manganese sulfate solution
[0087] will be 100m 3 MnSO 4 Adjust the solution to a concentration of 130g / L, add MnS at a rate of 2.5 kg per cubic meter of solution, and stir and react at 90°C for 3 hours to convert heavy metal impurity ions in the solution into insoluble sulfides. After that, press Separate by filtration, discard the filter residue, adjust the pH of the filtrate to 2.5-3.0 by sulfuric acid, add 2.5L of 27.5% by weight hydrogen peroxide per cubic meter into hydrogen peroxide, maintain slight boiling for 50 minutes under stirring, and use 2mol / L Ba(OH) 2 Neutralize to pH 5.0, separate by pressure filtration, and discard the filter residue.
[0088] (2) Oxidation reaction process
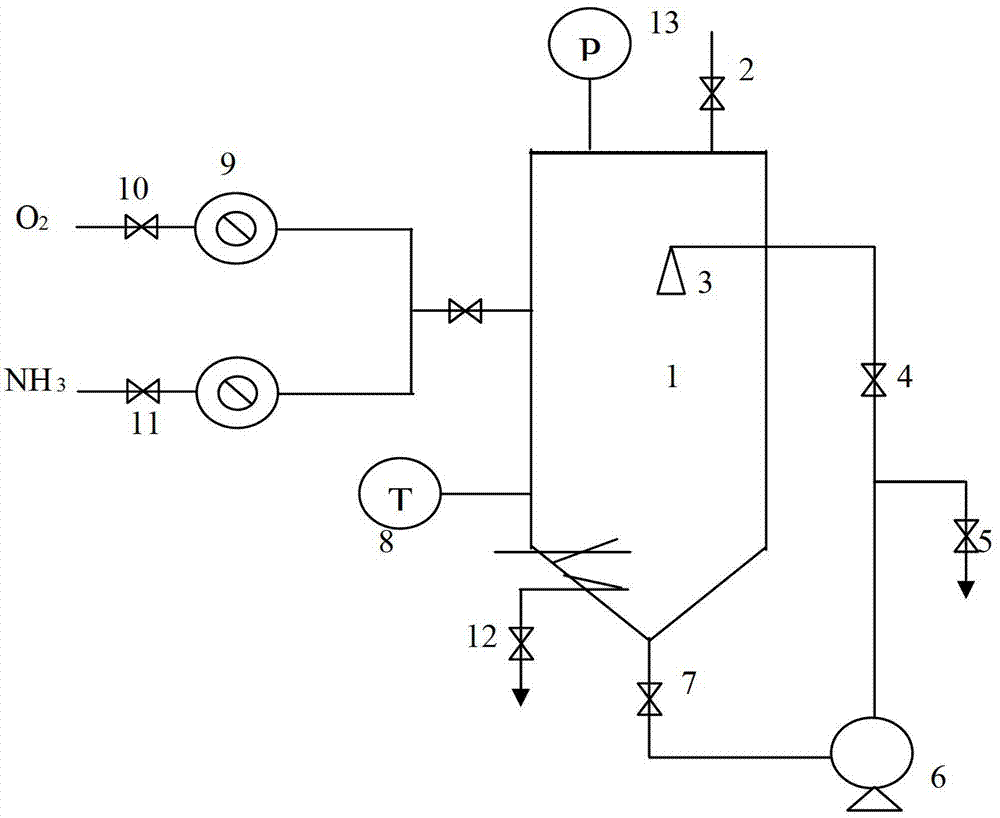
[0089] Place the above filtrate at the bottom of the reactor 1, cool it to 25-26°C through the coil on the reactor 1, open the exhaust valve 2, and open the oxygen valve 10 and the ammonia valve 11 with O 2 -NH 3 After the air in the tower is rep...
Embodiment 2
[0093] (1) Pretreatment process of manganese sulfate solution
[0094] will be 100m 3 MnSO 4 Adjust the solution to a concentration of 170g / L, add MnS at a rate of 3.0 kg per cubic meter of solution, and stir and react at 95°C for 4 hours to convert heavy metal impurity ions in the solution into insoluble sulfides. After that, press Separate by filtration, discard the filter residue, adjust the pH of the filtrate to 2.5-3.0 by sulfuric acid, add hydrogen peroxide at 2.5L 27.5% by weight per cubic meter of hydrogen peroxide, maintain slight boiling for 60 minutes under stirring, and use 2mol / L Ba(OH) 2 Neutralize to pH 5.5, separate by pressure filtration, and discard the filter residue.
[0095] (2) Oxidation reaction process
[0096] Place the above-mentioned filtrate at the bottom of the reactor 1, cool it to 27-28°C through the coil on the reactor 1, open the exhaust valve 2, and open the oxygen valve 10 and the ammonia valve 11 with O 2 -NH 3 After the air in the towe...
Embodiment 3
[0100] (1) Pretreatment process of manganese sulfate solution
[0101] will be 100m 3 MnSO 4 The solution was adjusted to a concentration of 200g / L, and MnS was added at a rate of 2.8 kg per cubic meter of solution, and stirred and reacted at 93°C for 4 hours to convert heavy metal impurity ions in the solution into insoluble sulfides, and then press Separate by filtration, discard the filter residue, adjust the pH of the filtrate to 2.5-3.0 by sulfuric acid, add hydrogen peroxide at 2.5L 27.5% by weight per cubic meter of hydrogen peroxide, maintain slight boiling for 55 minutes under stirring, and use 2mol / L Ba(OH) 2 Neutralize to pH 5.3, separate by pressure filtration, and discard the filter residue.
[0102] (2) Oxidation reaction process
[0103] Place the above filtrate at the bottom of the reactor 1, cool it to 28-30°C through the coil on the reactor 1, open the exhaust valve 2, and open the oxygen valve 10 and the ammonia valve 11 with O 2 -NH 3 After the air in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com