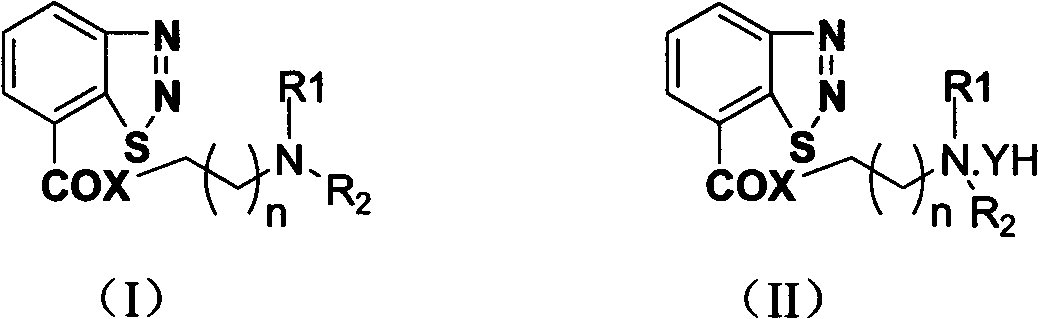
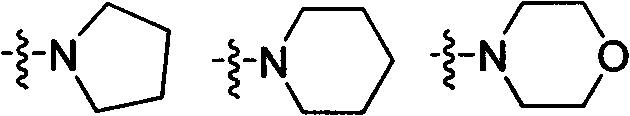
Preparation and plant activate antipathogen activity of benzo carboxylate derivatives of 1,2,3-thiadiazole
A technology of thiadiazole and derivatives, applied in the field of plant activation and disease resistance activity, can solve the problems of unsatisfactory water solubility and slow action speed, and achieve the effects of good water solubility, disease resistance activity, and damage prevention.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The following examples and bioassay results can be further used to illustrate the plant-activated disease resistance activity of the compounds of the present invention, but are not meant to limit the present invention.
[0046] Synthetic example
[0047] Synthesis of compound 1
[0048] 1. Preparation of benzo[1,2,3]-thiadiazole-7formyl chloride
[0049] Add 1.80 g (0.01 mol) of benzo[1,2,3]-thiadiazole-7-carboxylic acid and 10 ml of thionyl chloride into a 50 ml round-bottomed flask, heat and reflux at 80-90°C for 8 hours, Evaporate excess thionyl chloride under reduced pressure to obtain a brownish-yellow solid, and the intermediate benzo[1,2,3]-thiadiazole-7-formyl chloride is sealed and stored in a vacuum desiccator for subsequent use. The amount of the compound can be scale up or down accordingly.
[0050] 2. Compound 1: Synthesis of benzo[1,2,3]-thiadiazole-7-carboxylic acid (N,N-dimethyl)ethyl ester
[0051] Add 3.60 g (0.02 moles) of benzo[1,2,3]-thiadiazole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com