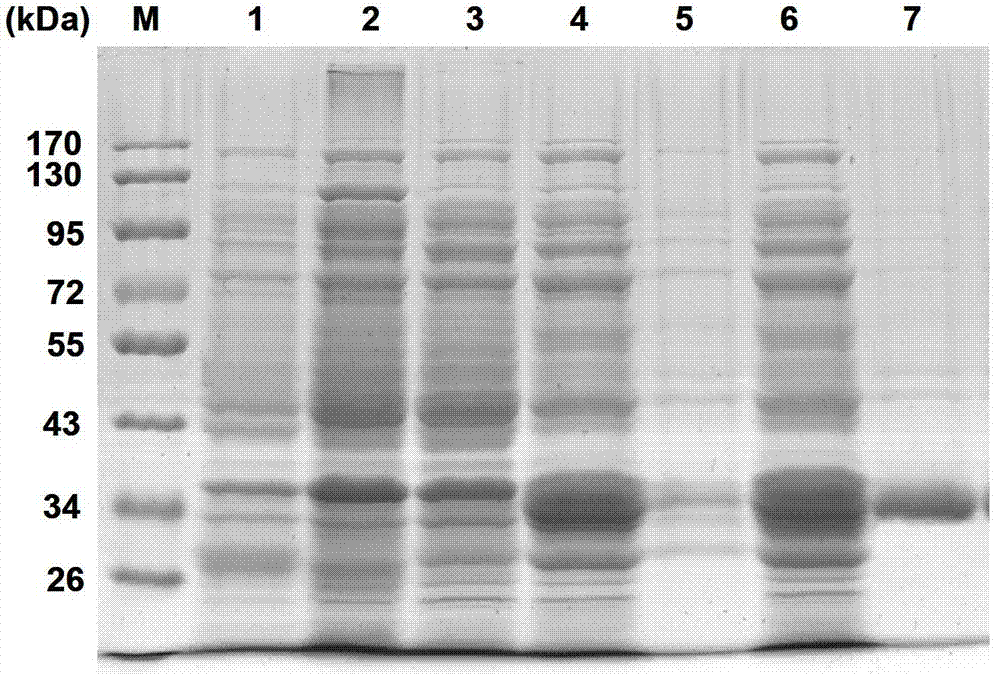
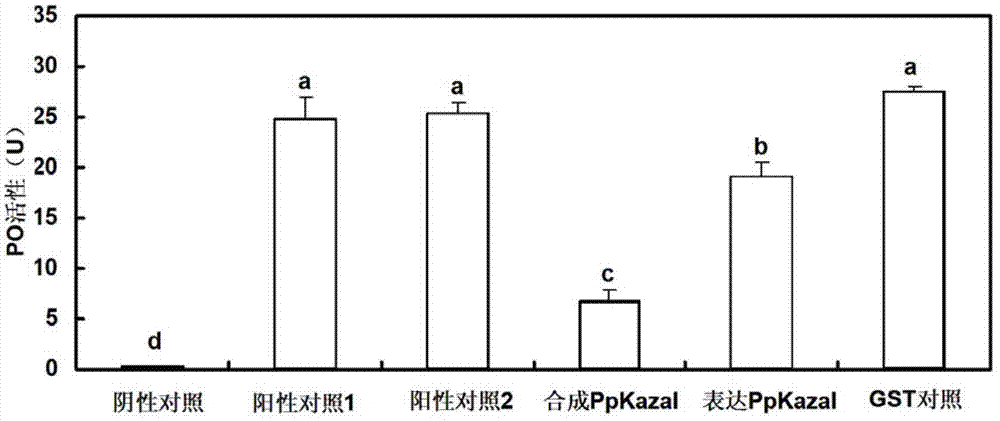
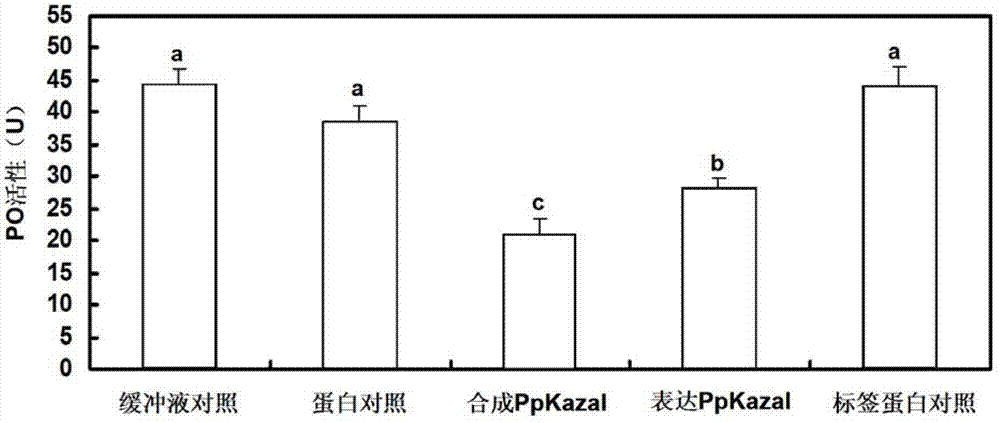
Pteromalus puparum venom Kazal serine proteinase inhibitor polypeptide and its application
A technology of serine protease and golden bee, applied in the direction of protease inhibitors, applications, animal/human peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1. PpKazal gene cloning of the Kazal-like serine protease inhibitor PpKazal from the chrysalis golden bee venom:
[0078] A pair of primers (forward primer Kaz-SP: 5'-CATGGCATTTGGATGTGAAG-3', reverse primer Kaz-AP: 5') were designed according to the partial sequence of the Kazal serine protease inhibitor gene obtained from the venom gland transcriptome data of Chrysalis chrysalis -TCTGTTCCATTCTCGGCCTT-3') Use the cDNA of the venom gland of Chrysalis chrysalis as a template to amplify by PCR to verify the transcriptome results, and a PCR fragment of about 200 bp was obtained by PCR. The PCR amplification system and amplification conditions are as follows:
[0079] The PCR amplification system is:
[0080]
[0081]
[0082] The amplification conditions are:
[0083]
[0084] PCR amplified products were separated by 1% agarose gel electrophoresis, purified by AxyAprep DNA gel recovery kit, ligated into pGEM?-T-easy vector (Promega) by TA cloning method, and posi...
Embodiment 2
[0135] 1. Construction of PpKazal gene plant binary expression vector
[0136] The cauliflower mosaic virus CaMV 35S promoter and NOS terminator on both sides of the GUS gene in the plant binary expression vector pBI121 were used to insert the PpKazal gene to form a complete expression framework. In this experiment, BamHI and SacI were selected as restriction sites to replace the GUS gene with PpKazal, so that the expression cassettes on both sides of the GUS gene were used to control the expression of the PpKazal gene in Arabidopsis plants.
[0137] Primers with BamHI and SacⅠ restriction sites were designed according to the ORF of the serine protease inhibitor of the venom of A. chrysalis, and the plant expression vector was constructed by PCR amplification using the cDNA of the venom gland of A. chrysalis as a template. The primer sequences are:
[0138] PpKazal-SP:TCG GGATCC TGTGAAGAAGAACAATGTCAAAAAA,
[0139] PpKazal-AP: CGC CTAACATTCCTCGTACTTGACAA,
[0140] in, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com