Method for detecting traditional Chinese medicine composition by using gas chromatography and fingerprint chromatography
A fingerprint and detection method technology, applied in the field of traditional Chinese medicine analysis, can solve the problem of single ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
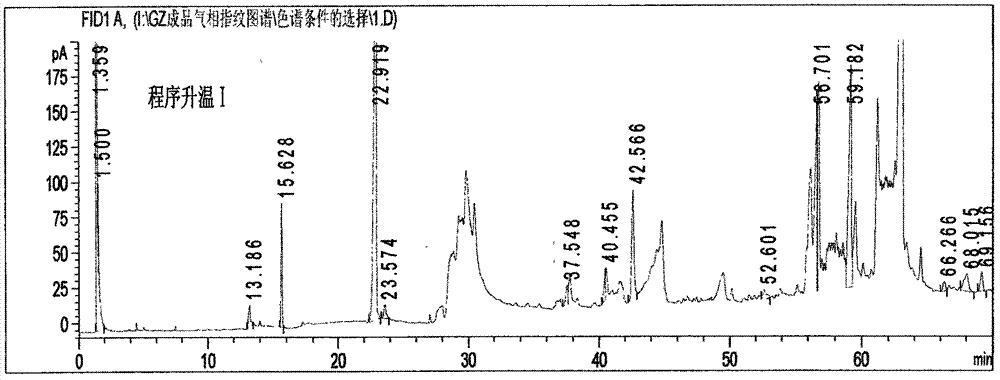
[0098] (1) Chromatographic conditions and system adaptability test: capillary column is selected; the temperature of the injection port is 220°C; the detector is a hydrogen flame ionization detector, and the detector temperature is 230°C; Raise the temperature at 15°C / min to 150°C and keep it for 5 minutes, then raise it to 240°C at a speed of 25°C / min and keep it for 13 minutes; the split ratio is 20:1; N 2 The flow rate is 0.5mL / min; the number of theoretical plates shall not be less than 3000 based on the calculation of the reference substance paeonol.
[0099] (2) Preparation method of the reference substance solution: take an appropriate amount of paeonol reference substance, weigh it accurately, add ether to prepare a solution containing 0.2 mg per 1 mL, and use paeonol as the reference substance.
[0100] (3) The preparation method of the test solution: take 2.0 g of Guizhi Fuling Pills, add 40 mL of water and 20 mL of ether, reflux in a water bath at 65 ° C for 1.0 h, ...
Embodiment 2
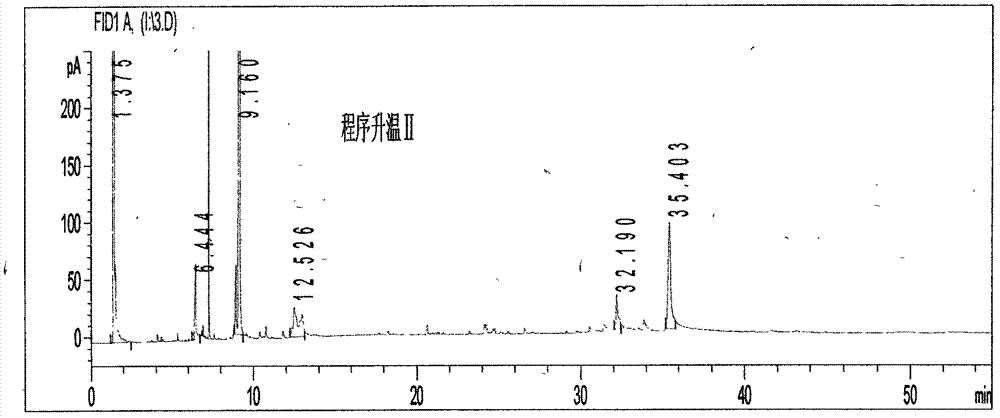
[0105] (1) Chromatographic conditions and system adaptability test: capillary column was selected; the temperature of the injection port was 225°C; the detector was a hydrogen flame ionization detector, and the detector temperature was 235°C; the column temperature was 75°C, and kept for 2.5min. Raise the temperature at 18°C / min to 155°C and keep it for 5.5 minutes, then raise the temperature at 28°C / min to 245°C and keep it for 13.5 minutes; the split ratio is 23:1; N 2 When the flow rate is 0.8mL / min, the number of theoretical plates shall not be less than 3000 based on the calculation of the reference substance paeonol.
[0106] (2) Preparation method of reference substance solution: take an appropriate amount of paeonol reference substance, weigh it accurately, add ether to prepare a solution containing 0.6 mg per 1 mL, and use paeonol as the reference substance.
[0107] (3) The preparation method of the test solution: take about 3.0 g of the cinnamon twig and poria cocos...
Embodiment 3
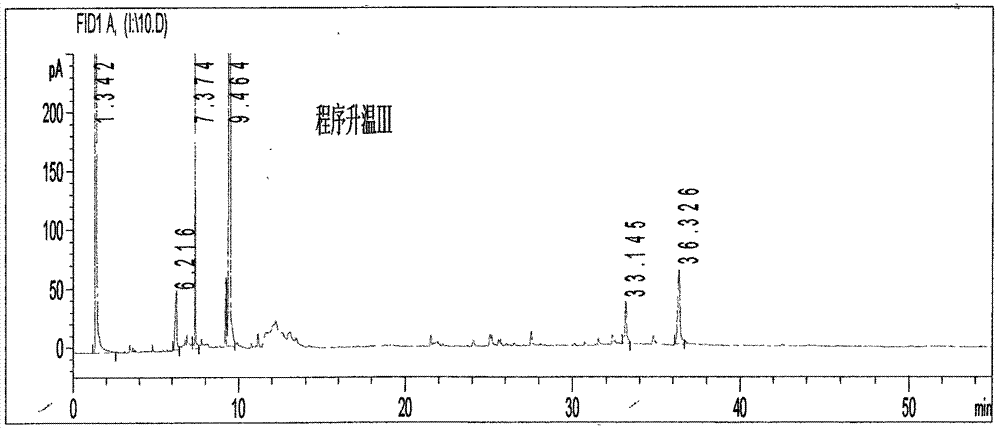
[0112] (1) Chromatographic conditions and system adaptability test: capillary column is selected; the temperature of the injection port is 230°C; the detector is a hydrogen flame ionization detector, and the detector temperature is 240°C; Raise the temperature at 20°C / min to 160°C and keep it for 6 minutes, then raise it to 250°C at a speed of 30°C / min and keep it for 14 minutes; the split ratio is 25:1; N 2 The flow rate is 1.0mL / min; the number of theoretical plates shall not be less than 3000 based on the calculation of the reference substance paeonol.
[0113] (2) Preparation method of reference substance solution: take an appropriate amount of paeonol reference substance, weigh it accurately, add ether to prepare a solution containing 1mg per 1mL, and use paeonol as the reference substance.
[0114] (3) The preparation method of the test solution: take 3.5g of Guizhi Fuling Capsules, add 50mL of water and 30mL of ether, reflux in a water bath at 75°C for 1.0h, separate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com