Synthesis method for key intermediate of silodosin
A technology of silodosin and a synthesis method, applied in the field of medicine, can solve problems such as unfavorable scale-up production, long synthesis route, cumbersome operation, etc., and achieve the effects of simplifying production operation, shortening synthesis steps, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
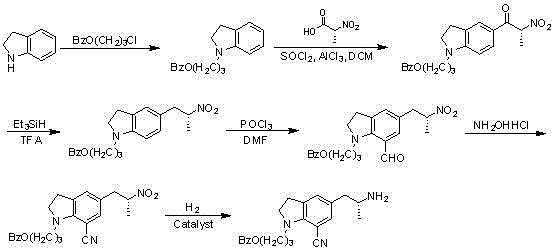
[0027] Embodiment 1, a kind of synthetic method of silodosin key intermediate, take indoline as starting material, first introduce benzoyloxypropyl group in the 1 position of indoline, synthetically obtain 1-(3 -benzoyloxypropyl) indoline, and then synthesize (R)-[1-(3-benzoyloxy) with (R)-2-nitropropionic acid through Friedel-Crafts acylation reaction Propyl)indolin-5-yl)]-2-nitropropyl-1-one, which is reduced by triethylsilane in trifluoroacetic acid to give (R)-[1-(3-benzoyl Oxypropyl) -5-(2-nitropropyl)]-indoline, and then introduce formyl at the 7-position through formylation to obtain (R)-1-[1-(3-benzoyl Oxypropyl)-5-(2-nitropropyl)7-formyl]indoline, and then react in hydroxylamine hydrochloride, pyridine, acetic anhydride to obtain (R)-1-[1-(3-benzene Formyloxypropyl)-5-(2-nitropropyl)7-cyano]indoline, and finally the target compound (R)-1-[1-(3-benzoyl) was obtained by catalytic hydrogenation oxypropyl)-5-(2-aminopropyl)7-cyano]indoline.
Embodiment 2
[0028] Embodiment 2, in the preparation method of the silodosin key intermediate described in embodiment 1: the synthesis of 1-(3-benzoyloxypropyl) indoline takes indoline as starting material, And benzoic acid, 1-chloro-3-bromopropane reaction in the system.
Embodiment 3
[0029] Example 3, in the preparation method of the silodosin key intermediate described in Example 1: (R)-[1-(3-benzoyloxypropyl)indoline-5-yl)]- The synthesis of 2-nitropropyl-1-ketone is to prepare (R)-2-nitropropionyl chloride by reacting (R)-2-nitropropionic acid with thionyl chloride, and then react with 1-(3- Benzoyloxypropyl) indoline is synthesized by Friedel-Crafts acylation reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com