Uracil or thymine derivative for treating hepatitis c
一种化合物、选自的技术,应用在药物组合、含有效成分的医用配制品、有机化学等方向,能够解决患者没有完成治疗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
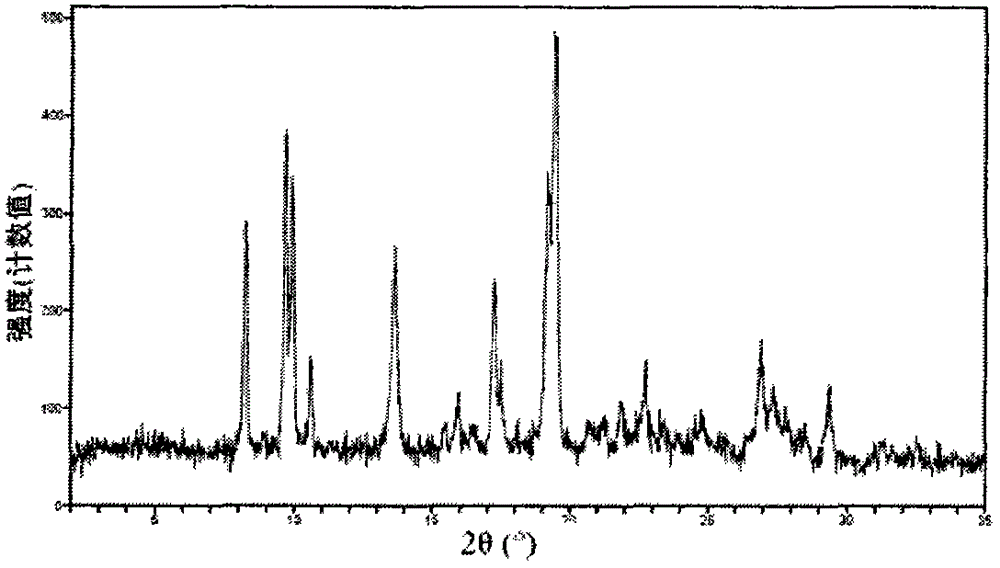
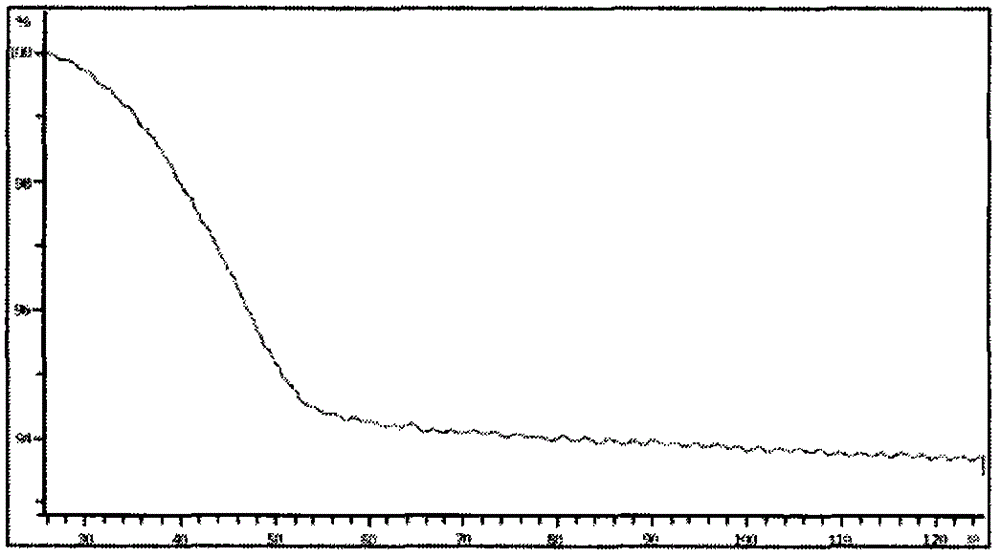
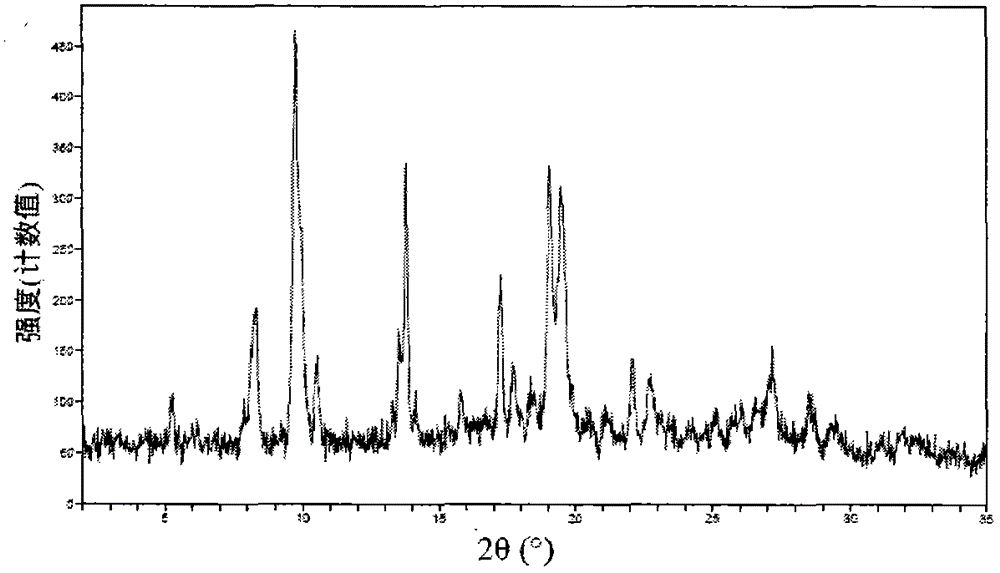
Image
Examples
Embodiment A
[1351] Example A. Preparation of (E)-N-(3-tert-butyl-5-iodo-4-methoxyphenylcarbamoyl)-3-methoxyacrylamide.
[1352]
[1353] Part A. Preparation of 2-tert-butyl-4-nitrophenol.
[1354] To a vigorously stirred solution of 2-tert-butylphenol (10 g, 66.6 mmol) in heptane (67 ml) was quickly added dropwise a solution of 70% nitric acid (4.25 ml, 66.6 mmol) diluted with water (4.25 ml). The resulting dark red / brown mixture was stirred vigorously for 2h. The suspended solid was collected by filtration, washed with hexane (300 mL), water (200 mL), and again with hexane (200 mL) to obtain cocoa powder, which was dried to constant weight (4.65 g, 35.6%).
[1355] Part B. Preparation of 2-tert-butyl-6-iodo-4-nitrophenol.
[1356] To the product of Part A (4.5 g, 23.05 mmol) dissolved in MeOH (120 ml) and water (30 mL), iodine monochloride (1.155 ml, 23.05 mmol) was added dropwise within 10 min. The mixture was stirred for 2h, diluted into 1L of water, and left overnight. The solid material w...
Embodiment B
[1363] Example B. Preparation of 1-(3-tert-butyl-5-iodo-4-methoxyphenyl)dihydropyrimidine-2,4(1H,3H)-dione.
[1364]
[1365] To the product of Example A (2.46g, 5.69mmol) in ethanol (50ml) was added 5.5mLH 2 SO 4 The mixture was heated at 110°C for 2.5h to obtain a transparent solution. The solution was cooled and diluted with 50 mL of water under stirring to obtain an off-white solid, which was collected by filtration, washed with water, and dried (2.06 g, 90%).
Embodiment C
[1366] Example C. Preparation of 1-(3-tert-butyl-5-iodo-4-methoxyphenyl)pyrimidine-2,4(1H,3H)-dione.
[1367]
[1368] Part A. Preparation of 2-tert-butyl-4,6-diiodophenol.
[1369] A solution of 2-tert-butylphenol (20.0 g, 133 mmol) in methanol (266 mL) was treated with sodium hydroxide pellets (6.39 g, 160 mmol). The mixture was stirred until all the sodium hydroxide was dissolved, and then cooled to -2°C in an ice-salt bath. Sodium iodide (15.0 g, 100 mmol) was added, and then a 10% sodium hypochlorite solution (45 mL, 73.3 mmol) was added dropwise at a rate that the temperature of the solution rose to no higher than 1.3°C. Repeat this process (3×) until a total of 60 g (400 mmol) of sodium iodide is added, and sodium hypochlorite solution is added, until the color of the solution changes from a light green-yellow color to a light ice tea color. This requires a ration of 16 mL of 180 mL of total sodium hypochlorite solution. Continue to cool at about 2°C, and add dropwise a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com