Flexible polyurethane?foam and manufacturing method thereof
A technology of flexible polyurethane and a manufacturing method, which is applied in the directions of packaged food, packaged item types, bandages, etc., can solve the problems of poor curability of flexible polyurethane foam, inability to meet thermoformability, etc., and achieve the effect of excellent discoloration resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] Hereinafter, specific examples of the present invention will be further described. Of course, the present invention is not limited to the examples.
[0062] Synthesis of allophanate modified polyisocyanate (A1) 1
Synthetic example 1
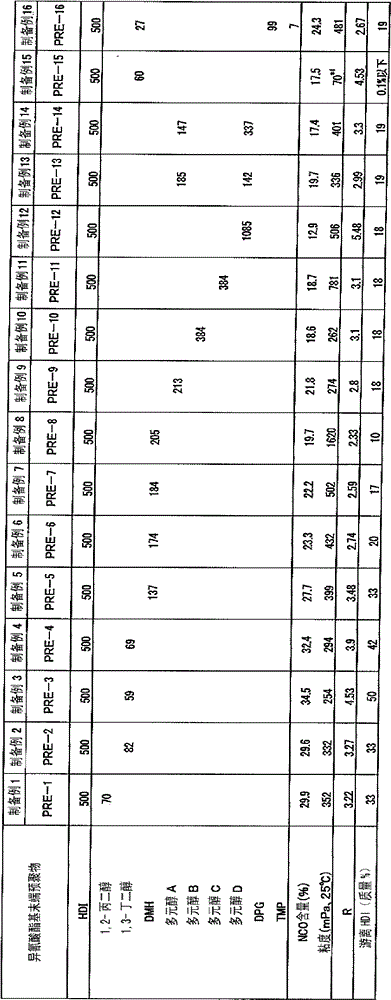
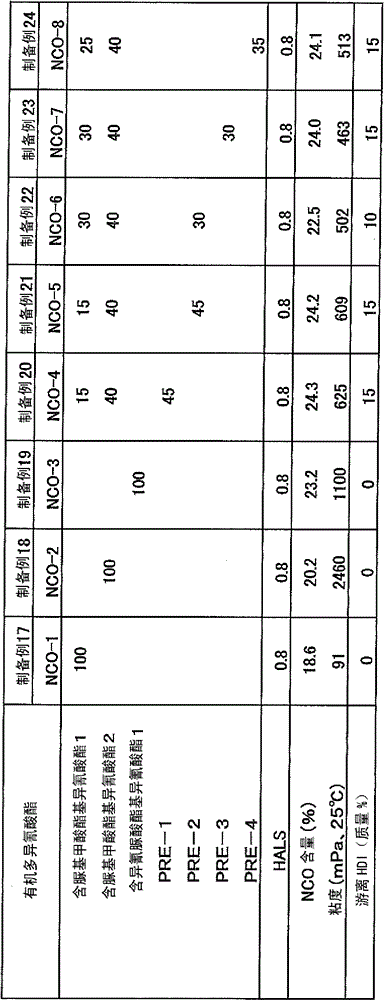
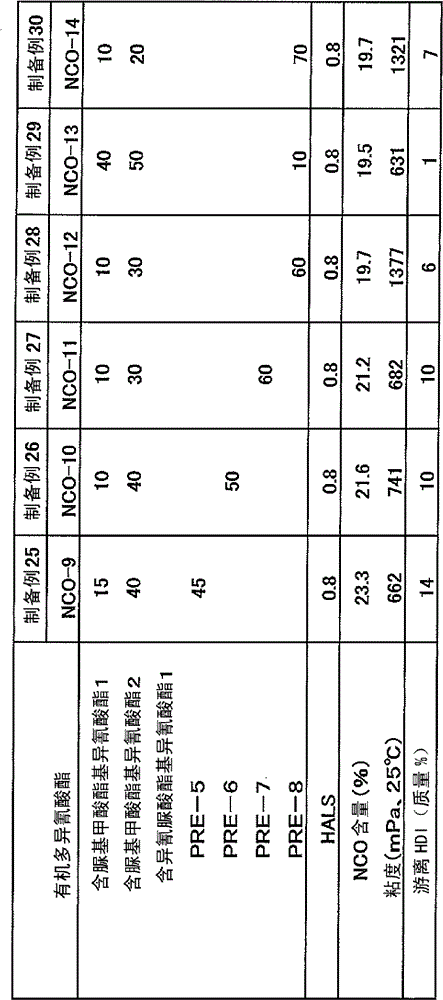
[0064] Put 950 g of hexamethylene diisocyanate (HDI) and 50 g of butanol into a reactor with a capacity of 1 L equipped with a stirrer, a thermometer, a cooler, and a nitrogen gas introduction pipe, and perform a urethanization reaction at 90°C for 2 hours . The reaction product was analyzed by FT-IR, and the hydroxyl group disappeared. Next, 0.1 g of zirconyl octoate was charged and reacted at 90° C. for 3 hours. With FT-IR and 13 As a result of C-NMR analysis of the reaction product, the carbamate group disappeared. Next, 0.11 g of JP-508 (manufactured by Johoku Chemical Co., Ltd.) was charged, and the reaction was stopped at 50° C. for 1 hour. The isocyanate content of the reaction product after stopping the reaction was 40.14%. The reaction product was subjected to thin-film distillation at 140°C and 40Pa to obtain a "synthetic acid" with an isocyanate content of 18.6%, a viscosity of 91 mPa·s at 25°C, a free hexamethylene diisocyanate content of 0.1%, and a color numb...
Synthetic example 2
[0067] By the same method as Synthetic Example 1, drop into 950g hexamethylene diisocyanate (HDI), 50g 1,3-butanediol, after reaction, obtain " allophanate modified polyisocyanate-2 " (isocyanate content 20.2%, the viscosity at 25°C is 2460mPa·s, the free hexamethylene diisocyanate is 0.1%, and the color number is 20APHA).
[0068] Synthesis of Isocyanates Containing Isocyanurate Groups
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com