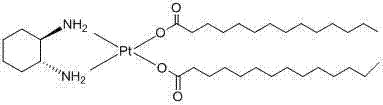
New method for synthesizing anti-tumor drug miboplatin
A technology of miplatin and substances, which is applied in the field of preparation of platinum anti-tumor drug miplatin, which can solve the problems of greater harm to the human body and the environment, excessive silver ions, etc., and achieve the effects of short production cycle, avoiding damage, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 8.3gK 2 PtCl 4 , dissolved in water and filtered off the insoluble matter, weighed 7gNaNO 2 , dissolved in water and added to K 2 PtCl 4 In solution, react at 70°C for 6h to obtain light yellow K 2 Pt(NO 2 ) 4 solution. Weigh 2.8g (1R,2R)-1,2-cyclohexanediamine, dissolve it in water and add it dropwise to K 2 Pt(NO 2 ) 4 In the solution, after reacting at room temperature for 5 h, the obtained solid was filtered and dried to obtain 7.3 g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum(II) solid. Weigh 5g of hydrazine sulfate and dissolve it in 100ml of hot water, add 7.3g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum (II) solid, heat in a water bath for 3 hours and then cool , a small amount of solid was filtered to obtain a pale yellow hydrolyzed solution. Take by weighing 10g sodium tetradecanoic acid, dissolve it with 100ml n-butanol and add it dropwise in the hydrolysis solution, react at room temperature for 7h, filter the w...
Embodiment 2
[0026] Weigh 8.3gK 2 PtCl 4 , dissolved in water and filtered off the insoluble matter, weighed 7.5gNaNO 2 , dissolved in water and added to K 2 PtCl 4 In solution, react at 65°C for 6h to obtain light yellow K 2 Pt(NO 2 ) 4 solution. Weigh 2.3g (1R,2R)-1,2-cyclohexanediamine, dissolve it in water and add it dropwise to K 2 Pt(NO 2 ) 4 In the solution, after reacting at room temperature for 5 h, the obtained solid was filtered and dried to obtain 7.1 g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum(II) solid. Weigh 6g of hydrazine sulfate and dissolve it in 100ml of hot water, add 7.1g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum (II) solid, heat in a water bath for 4h and then cool , a small amount of solid was filtered to obtain a pale yellow hydrolyzed solution. Weigh 12g of sodium tetradecanoic acid, dissolve it with 120ml of n-butanol and add it dropwise to the hydrolysis solution. After reacting at room temperature for 7 hours, filter...
Embodiment 3
[0028] Weigh 8.3gK 2 PtCl 4 , dissolved in water and filtered off the insoluble matter, weighed 7.5gNaNO 2 , dissolved in water and added to K 2 PtCl 4 In solution, react at 60°C for 7h to obtain light yellow K 2 Pt(NO 2 ) 4 solution. Weigh 2.3g (1R,2R)-1,2-cyclohexanediamine, dissolve it in water and add it dropwise to K 2 Pt(NO 2 ) 4 In the solution, after reacting at room temperature for 7 hours, the obtained solid was filtered and dried to obtain 7.5 g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum(II) solid. Weigh 6.5g of hydrazine sulfate and dissolve it in 100ml of hot water, add 7.5g of cis-dinitroso-(1R,2R)-1,2-cyclohexanediamine platinum (II) solid, and heat in a water bath for 3.5h After cooling, a small amount of solid was filtered to obtain a pale yellow hydrolyzed solution. Weigh 12g of sodium tetradecanoic acid, dissolve it with 150ml of n-butanol and add it dropwise to the hydrolysis solution. After reacting at room temperature for 8 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com