Chloro-substituted polyetherimides having improved relative thermal index
A technology of polyetherimide and polyimide, applied in the direction of organic chemistry, etc., can solve the problems of non-commercial application, polyetherimide lowering of relative thermal index properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-54
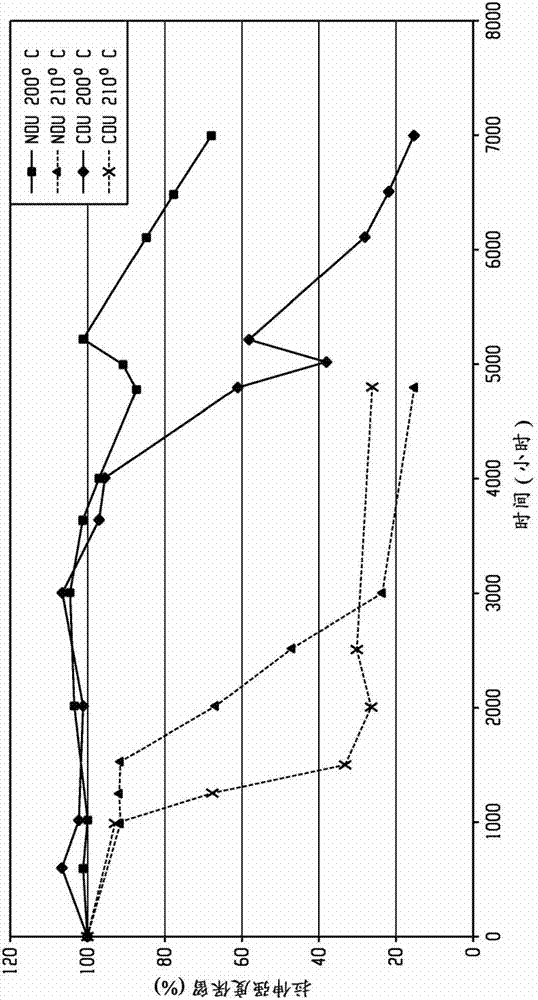
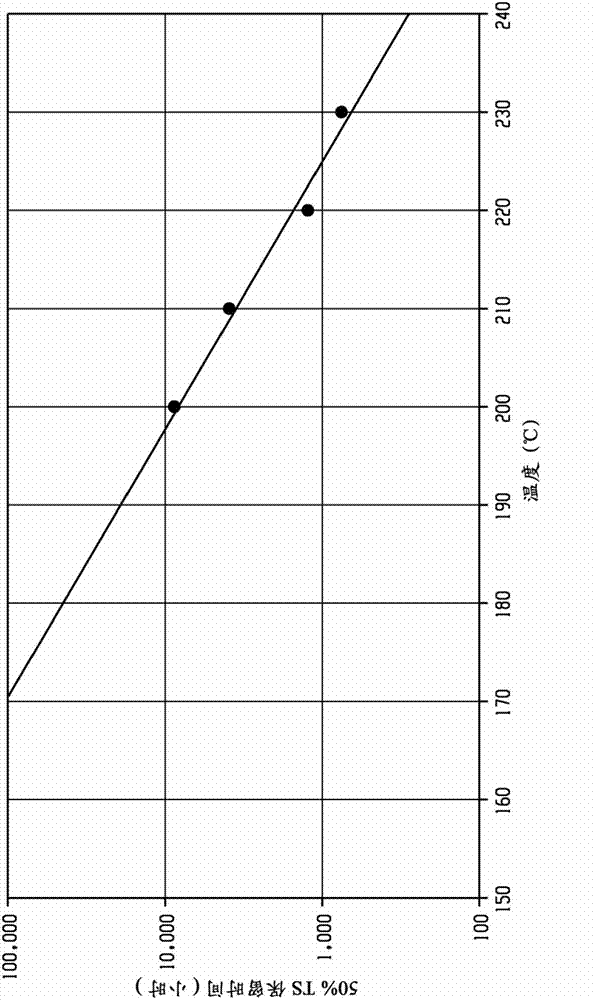
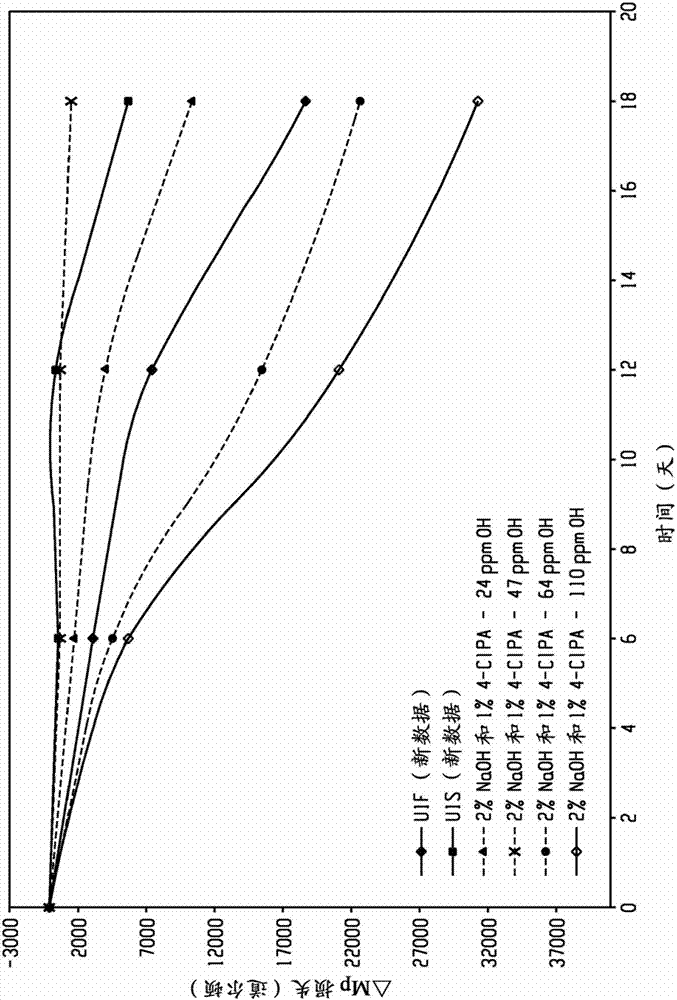
[0103] In the following examples, the prepared polyetherimide was derivatized with a phosphorylation reagent, and then tested for the hydroxyl end group content by phosphorus 31 nuclear magnetic resonance (P31 NMR). Relative thermal index (RTI) ratings of polyetherimides are measured by the "Accelerated Thermal Aging Test Method" described further below, or by the "Underwriter's Laboratory Relative heat index test method UL746B determination. The molecular weight of the polyetherimide prepared in the embodiment is determined by gel permeation chromatography (GPC) using Polymer Laboratory mixed bed C column, dichloromethane as eluent, and polystyrene narrow molecular weight standard sample to determine Mp (peak molecular weight), Mn (number average molecular weight), and Mw (weight average molecular weight) of the material.
[0104] Techniques and Procedures
[0105] Underwriter's Laboratory Relative heat index test: the control polyetherimide and the polyetherimide of the...
Embodiment 1-9
[0113] Examples 1-9 investigate the effect of the presence of different bases on the hydroxyl end group content of polyetherimides during polymerization. The following examples include bisphenol A disodium salt and 1,3-bis(N-(4-chlorophthalimido))benzene (ClPAMI) in the presence of hexaethylguanidine chloride (HEGCl) catalyst polymerization. All polymerizations were carried out in o-dichlorobenzene (ODCB). Reactions were performed on laboratory scale.
[0114] Bisphenol A disodium salt was isolated and prepared as follows. To a 1 liter (L) round bottom flask was added a slurry of bisphenol A disodium salt in o-dichlorobenzene (ODCB). ODCB was removed by rotary evaporator (150 °C, full (2) filled, detached from the rotary evaporator, and placed in a vacuum oven for 3 days (130°C, full (2 Save it.
[0115] ClPAMI was isolated and prepared as follows. A sample of the ClPAMI slurry in ODCB was filtered using a Buchner funnel. The solid was washed successively with warm ODCB ...
Embodiment 10-12
[0123] Examples 10-12 investigate the effect of base addition point on the hydroxyl end group content of polyetherimides. K 3 PO 4 Used as a base. The base was added to bisphenol A disodium salt, the ClPAMI slurry was added and during polymerization.
[0124] The following example involves the polymerization of bisphenol A disodium salt and 1,3-bis(N-(4-chlorophthalimido))benzene (ClPAMI) in the presence of hexaethylguanidine chloride (HEGCl) catalyst . ClPAMI is rich in 4-monoamines. On a stoichiometric basis, amine-rich ClPAMI performed equally well in polymerization with bisphenol A disodium salt. All polymerizations were carried out in o-dichlorobenzene (ODCB). Reactions were performed on laboratory scale as described above.
[0125] Bisphenol A disodium salt was isolated and prepared as described above in Examples 1-9.
[0126] 4-Monoamine (4-MA) enriched ClPAMI was prepared as follows. Add m-phenylenediamine (2.743g, 25.365mmol), 4-chlorophthalic anhydride (4-Cl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative thermal index | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com