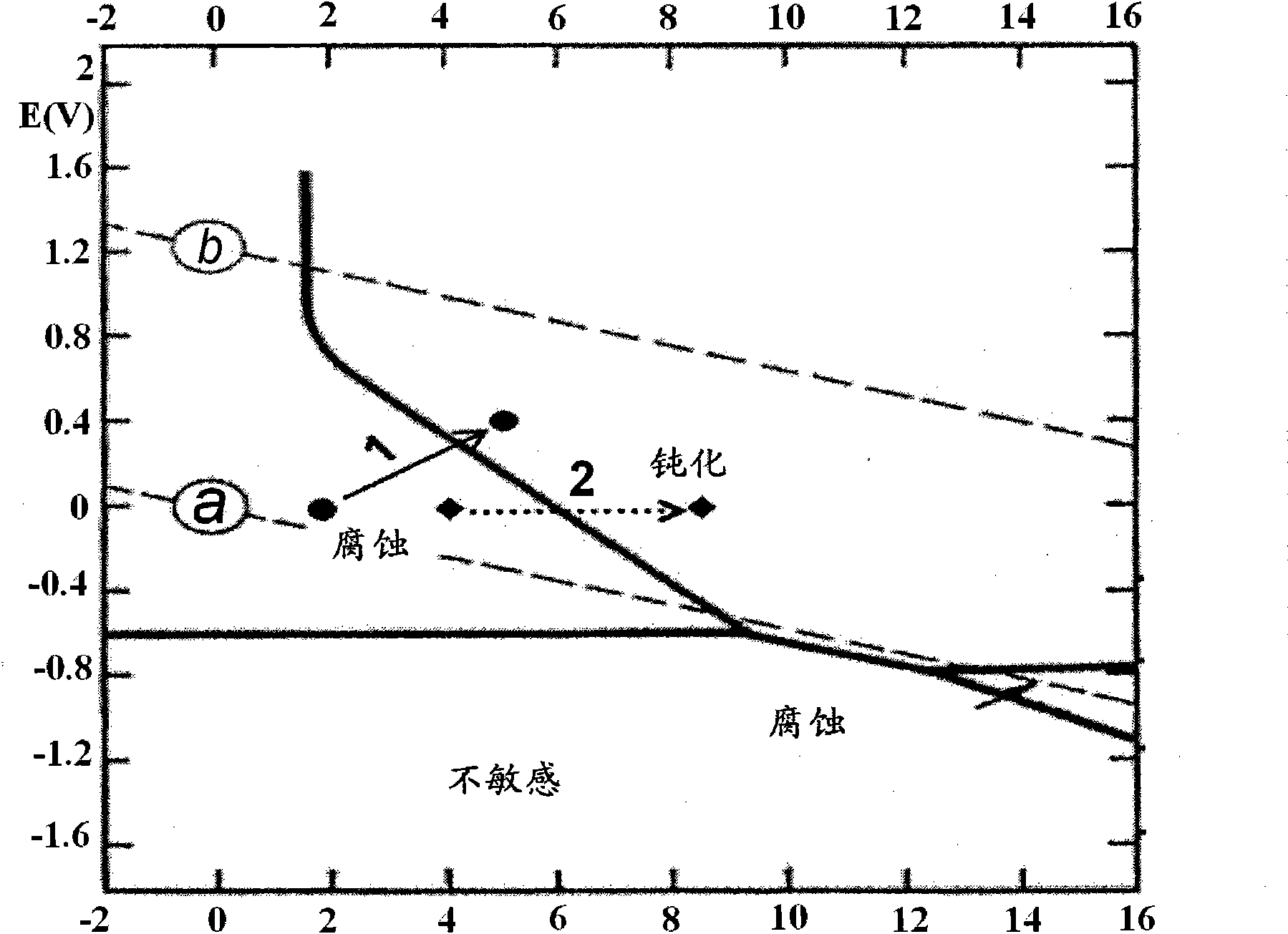
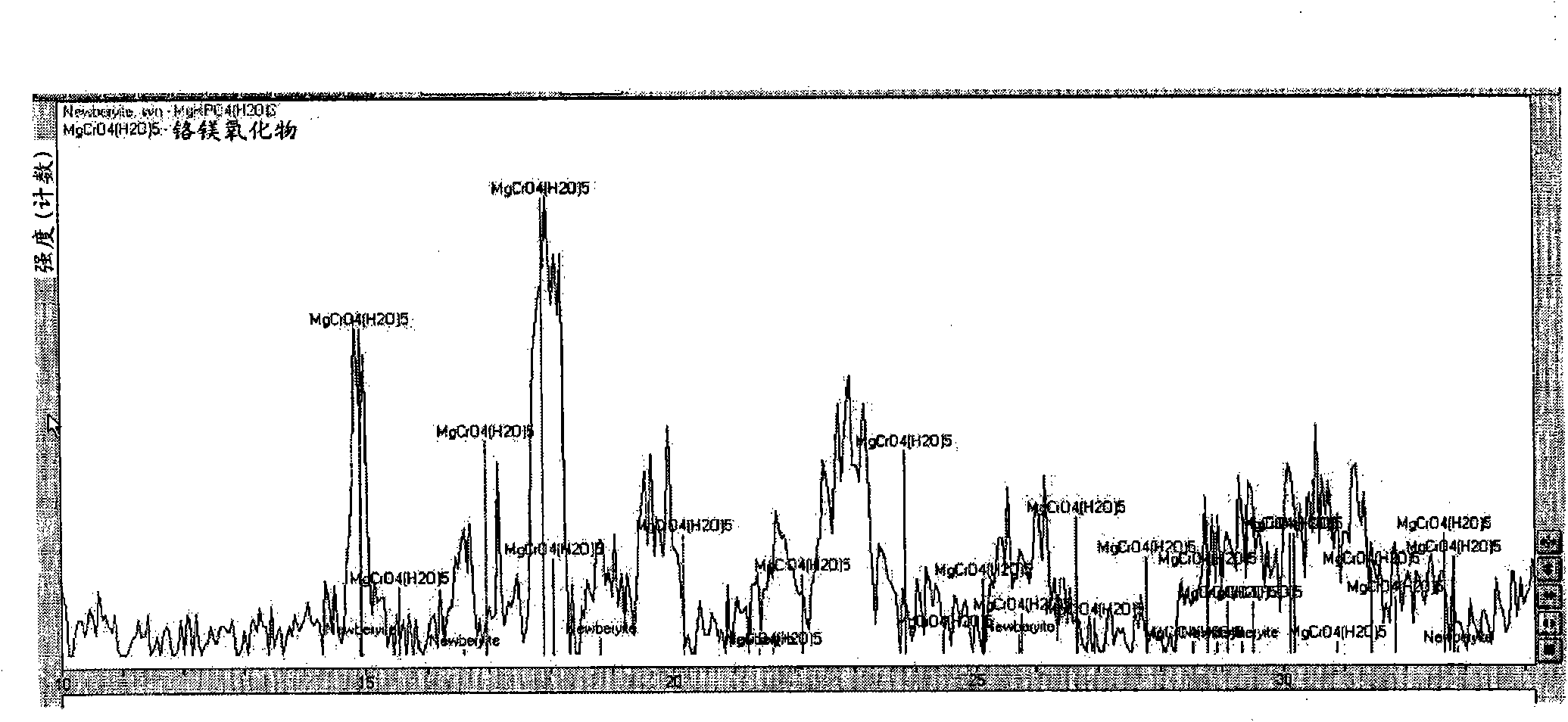
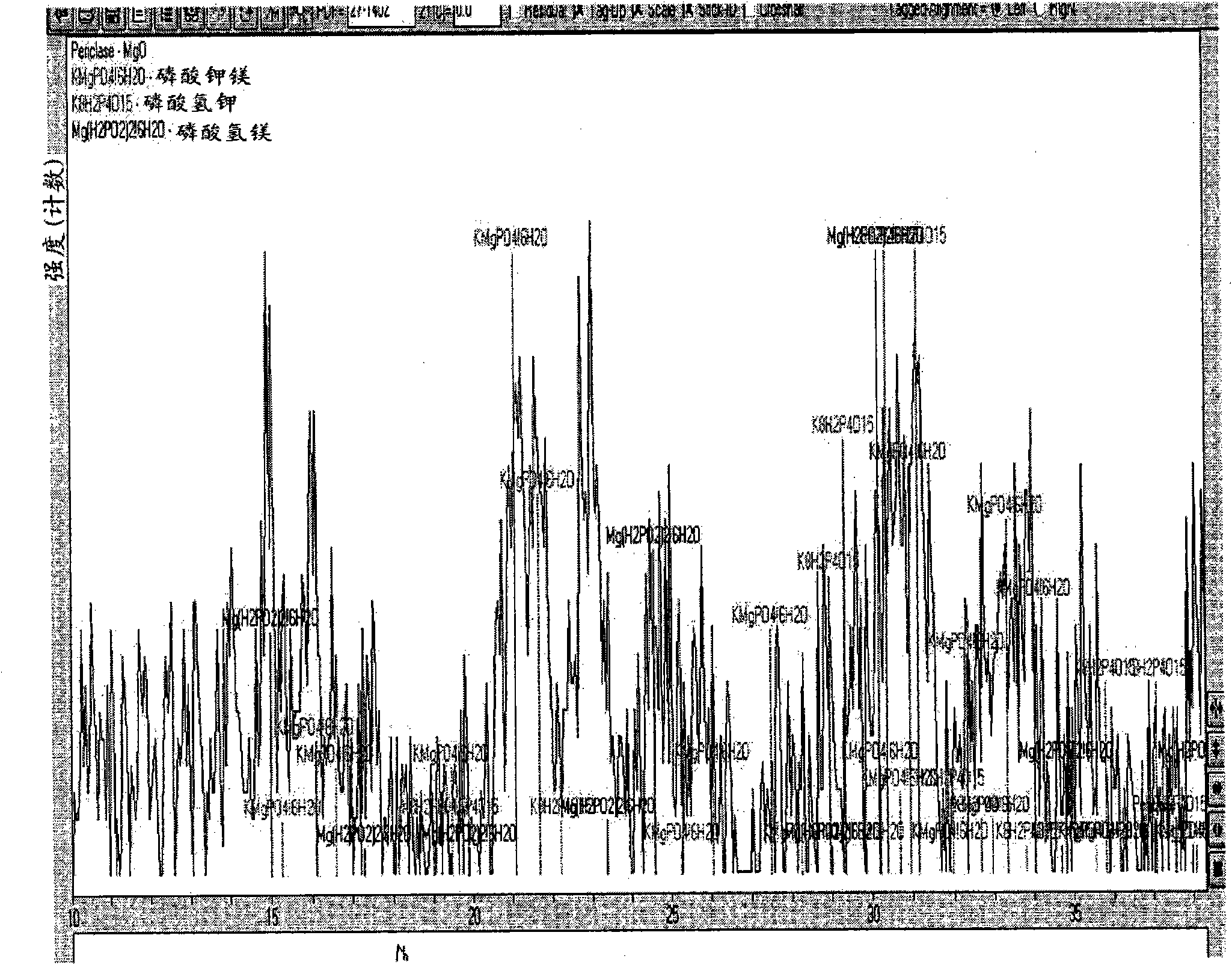
Inorganic phosphate corrosion resistant coatings
An anti-corrosion, acid phosphate technology, used in anti-corrosion coatings, metal material coating processes, cement coatings, etc., can solve problems such as toxic gas, environmental waste disposal, and wear resistance of passivation layers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: MHP-based corrosion protection layer
[0076] In this example, MHP (Mg(H 2 PO 4 ) 2 2H 2 O), and calcium silicate and alumina are added as fillers to form a slurry. The amount of water used to dilute the MHP-based material can vary depending on the amount of water contained in the starting material (MHP-based materials are difficult to dry when prepared and therefore usually contain some water). Preferably, the dilution water should be added in an amount corresponding to about 20% by weight of the MHP. The amounts of calcium silicate and alumina added as fillers to form the slurry can also vary. In this example, for every 100 g of MHP, 80 g of calcium silicate and 60 g of alumina were added. For every 100 g of MHP, 96 g of MgO were added to the mixture. Calcium silicate and alumina were mixed separately for 10 minutes. When adding the MgO, monitor the temperature of the paste and mix until the temperature reaches about 85°F. The paste was then applie...
Embodiment 2
[0077] Example 2: Anti-corrosion layer on corroded steel surface
[0078] In this example, a calcium silicate-containing MKP-based formulation prepared as a paste was applied to a rusted steel surface. The MKP paste is formed by mixing 1 part through-calcined magnesia (calcined at a temperature greater than about 1300°C), 3 parts potassium dihydrogen phosphate, and 6 parts calcium silicate. To this powder mixture was added 2 parts of water to provide a paste. As mixing continues, initially the paste will cool a few degrees, indicating dissolution of the monobasic potassium phosphate; but the temperature will begin to rise as the magnesium oxide begins to react. Mixing was continued until the temperature of the paste rose to about 85°F, and at the same time, the paste was applied to the rusted steel surface. Upon curing, the top (excess) portion of the coating can be easily removed. However, this hardened layer also removes the corrosion (rust) layer from the plate. Unexpec...
Embodiment 3
[0079] Embodiment 3: Iron oxide base anticorrosion paint
[0080] In this example, 165 g of MHP material was dissolved in 168 g of water by mixing and stirring for about 1 hour. Add 16.5g wollastonite (CaSiO 3 ). The resulting paste was stirred and mixed for about 35 minutes before adding 200 g of hematite (Fe 2 o 3 ) and the paste was continued to stir and mix for about 15 minutes. Then, add 5g magnetite (Fe 3 o 4 ) and the paste was continued to stir and mix for about 10 minutes. Then, the resulting paste was coated on the surface of a polished mild steel plate. Hardens very slowly. There is no detectable heat during curing, however once hardened the coating adheres to the steel surface and cannot be easily removed. This coating provides excellent corrosion protection for steel. On this surface, a second layer of phosphate cement may optionally be added as described in Example 4 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com