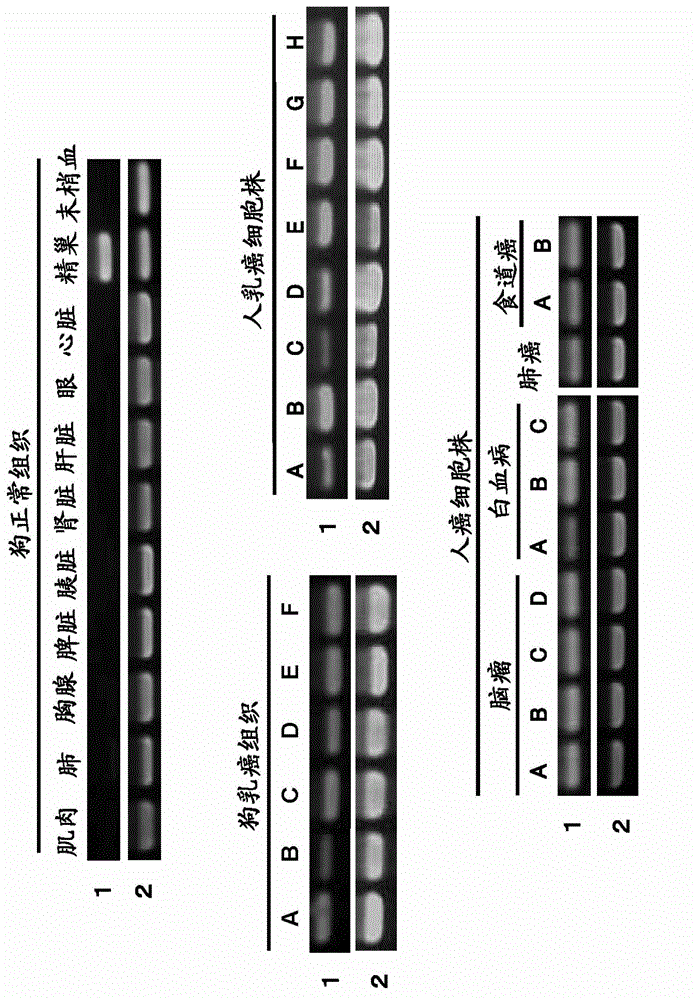
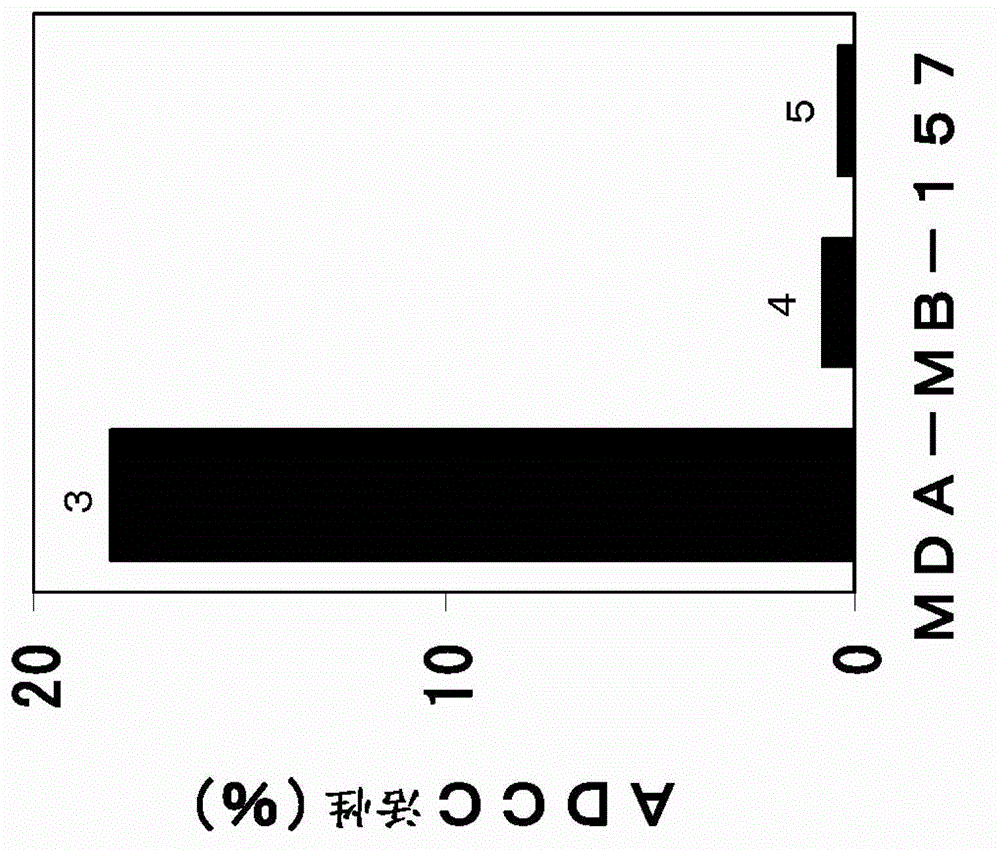
Pharmaceutical composition for treating and/or preventing cancer
A technology of variable regions and antibodies, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation of the antigen, the preparation of the antibody, and the pharmaceutical composition related to the present invention will be described below.
[0047]
[0048] The protein or its fragment used as a sensitizing antigen for obtaining the antibody against CAPRIN-1 used in the present invention can be derived from humans, dogs, cows, horses, mice, rats, chickens, etc. The types of animals are not limited. However, the protein or fragment thereof is preferably selected in consideration of suitability for mother cells used in cell fusion, and generally, mammalian-derived proteins are preferred, and human-derived proteins are particularly preferred. For example, when CAPRIN-1 is human CAPRIN-1, human CAPRIN-1 protein and / or partial peptides thereof, cells expressing human CAPRIN-1, etc. can be used.
[0049] The base sequence and amino acid sequence of human CAPRIN-1 and its homologues can be obtained, for example, by accessing GenBank (US NCBI), using algor...
Embodiment 1
[0180] Example 1 Identification of a new cancer antigen protein using the SEREX method
[0181] (1) Preparation of cDNA library
[0182] Total RNA was extracted from the testis tissue of healthy dogs by the Acid guanidium-Phenol-Chloroform method (Acid guanidium-Phenol-Chloroform method), and the Oligotex-dT30 mRNA purification kit (manufactured by Takara Shuzo Co., Ltd.) was used to purify the polysaccharide A RNA.
[0183] The thus obtained mRNA (5 µg) was used to synthesize a dog testis cDNA phage library. The cDNA phage library was prepared using cDNA Synthesis Kit, ZAP-cDNA Synthesis Kit, and ZAP-cDNA GigapackIII Gold Cloning Kit (manufactured by STRATAGENE), and the library was prepared according to the instructions attached to the kit. The size of the cDNA phage library produced was 7.73×10 5 pfu / ml.
[0184] (2) Screening of cDNA library using serum
[0185] The dog testis cDNA phage library prepared above was used for immune screening. Specifically, infect the h...
Embodiment 2
[0213] The preparation of embodiment 2 human CAPRIN-1
[0214] (1) Preparation of recombinant protein
[0215] Based on the gene of SEQ ID NO: 1 obtained in Example 1, the recombinant protein of human CAPRIN-1 was prepared by the following method. PCR was carried out as follows: 1 μl of cDNA whose expression could be confirmed by RT-PCR using the cDNA of various human tissues and cells prepared in Example 1, two primers containing the restriction enzyme cutting sequence of SacI and XhoI (sequence number 38 and 39) each of 0.4 μM, 0.2 mM dNTP, and 1.25 U PrimeSTAR HS polymerase (manufactured by Takara Shuzo Co., Ltd.)) was added to make the total amount of 50 μl of each reagent and accompanying buffer, and a Thermal Cycler (BIO RAD, Inc. system), the cycle of 98°C-10 seconds and 68°C-2.5 minutes was repeated 30 times. In addition, the above-mentioned two types of primers are primers for amplifying the region encoding the entire length of the amino acid sequence of SEQ ID NO: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com