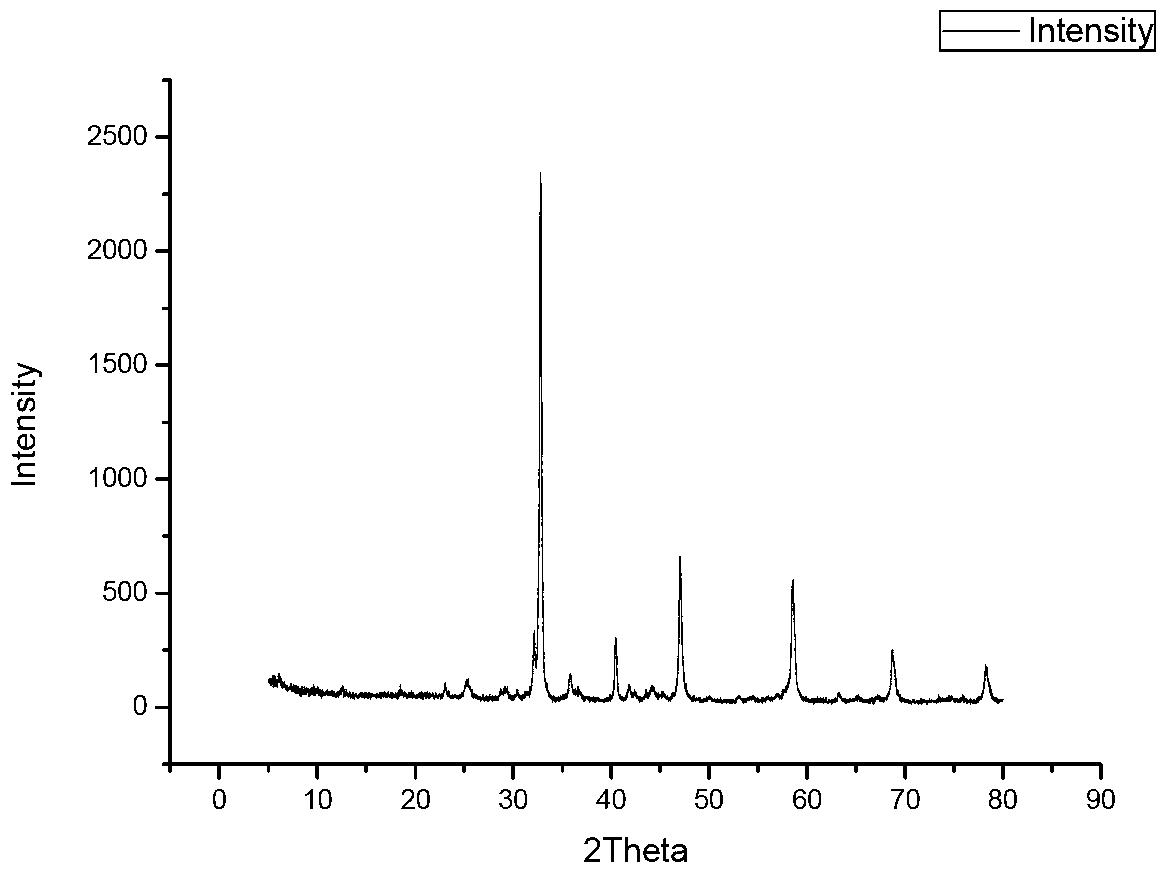
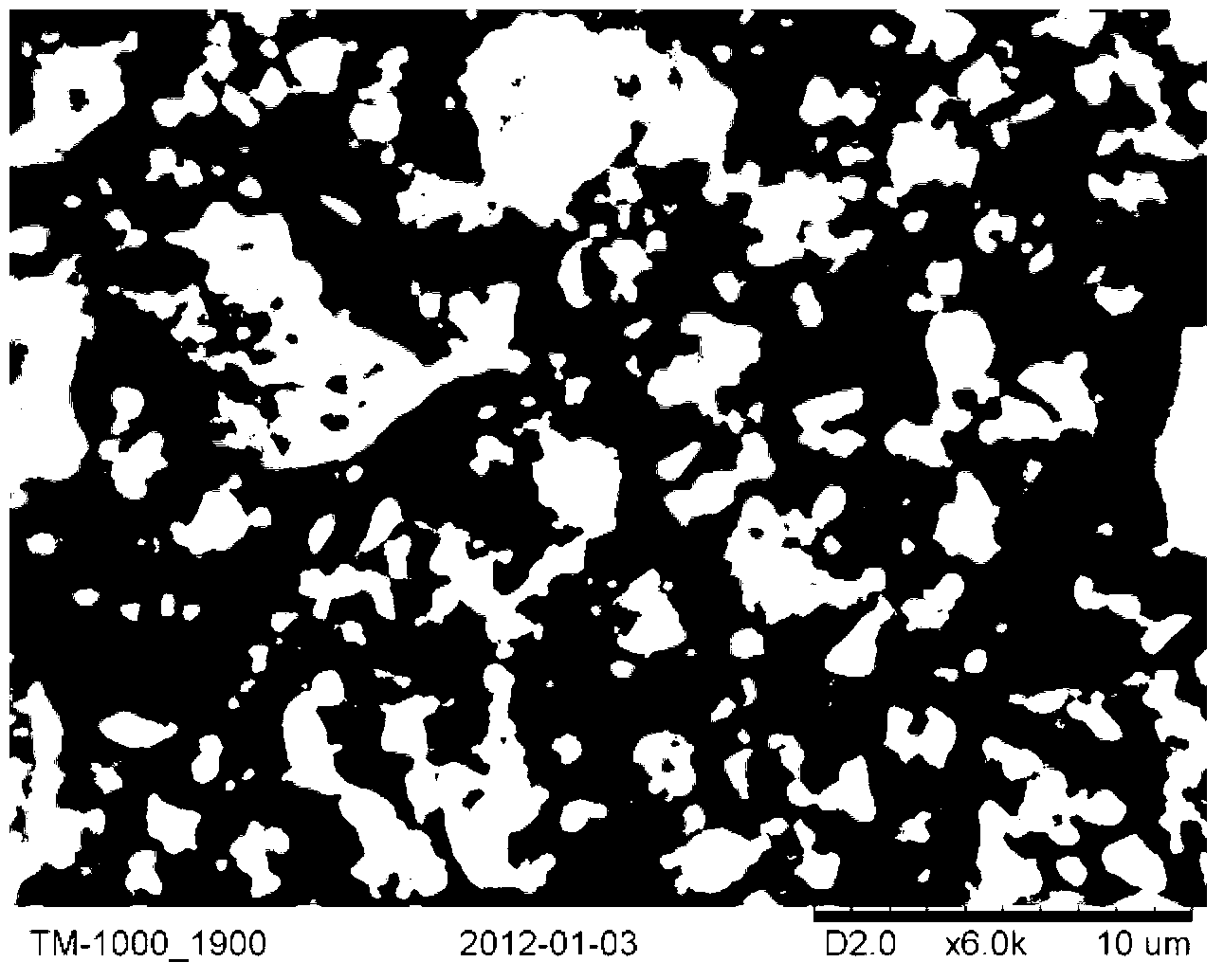
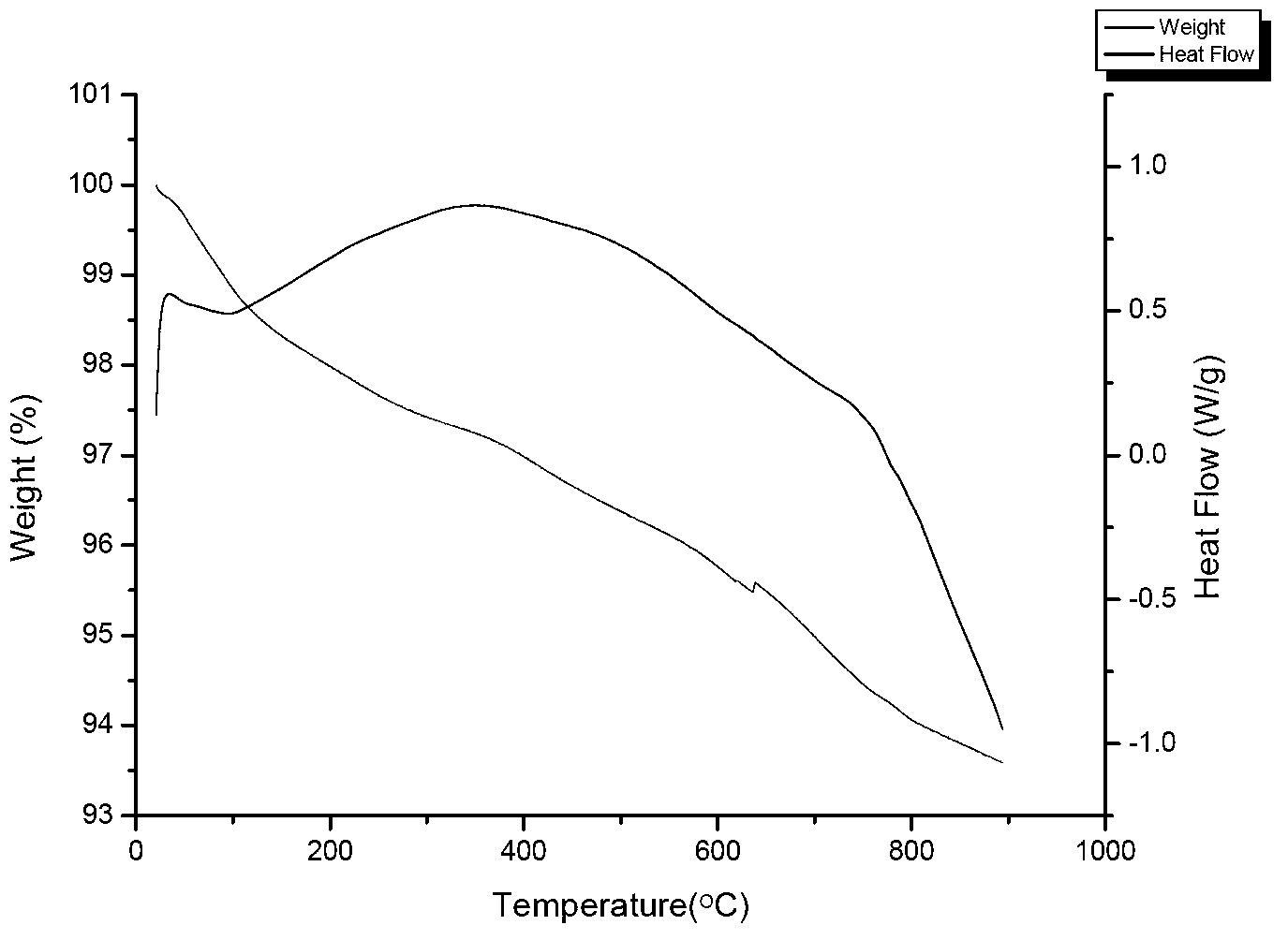
Preparation method of perovskite type photocatalyst and product thereof
A photocatalyst, perovskite-type technology, applied in the field of preparation method of perovskite photocatalyst and its products, can solve problems such as low degradation rate, and achieve the effects of convenient operation, simple process and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] SrFe 0.5 co 0.5 o 3-δ Preparation of photocatalyst
[0045] Weigh 1.0mol Sr(NO 3 ) 2 , 0.03mol Co(NO 3 ) 2 ·6H 2 O, 0.03mol Fe(NO 3 ) 3 9H 2 O, 0.10mol citric acid, put into a beaker, add 300ml of distilled water, stir until dissolved, cover the opening of the beaker with a watch glass, seal it, then place the beaker on a constant temperature magnetic stirrer for heating and stirring, and heat to 92°C , stirred at constant temperature for 3.5 hours, opened the opening of the beaker, and continued to heat until the water evaporated to form a colloid, and placed the colloid in a blast drying oven for 18 hours to keep warm and dry at a temperature of 110°C;
[0046] The dried sample was taken out, ground into fine powder, put into an alumina crucible, and calcined. During calcination, the heating rate is 5°C / min, the temperature is raised to 185°C, and the temperature is kept for 1.2 hours; the temperature is continued to be raised to 400°C, and the temperature ...
Embodiment 2
[0055] Ba 0.2 Sr 0.8 Fe 0.5 co 0.5 o 3-δ Preparation of photocatalyst:
[0056] Weigh 0.02mol Ba(NO 3 ) 2 , 0.08mol Sr(NO 3 ) 2 , 0.05mol Co(NO 3 ) 2 ·6H 2 O, 0.05mol Fe(NO 3 ) 3 9H 2 O, 0.15mol citric acid, put into a beaker, add 350ml of distilled water, stir until dissolved, cover the opening of the beaker with a watch glass, seal it, then place the beaker on a constant temperature magnetic stirrer for heating and stirring, and heat to 90°C , Stir at constant temperature for 3 hours, open the opening of the beaker, continue to heat until the water evaporates to form a colloid, place the colloid in a blast drying oven for 20 hours and keep it dry for 20 hours at a temperature of 120°C;
[0057] (2) Ba 0.2 Sr 0.8 Fe 0.5 co0.5 o 3-δ Calcination of photocatalyst: Take out the dried sample, grind it finely, put it into an alumina crucible, and carry out calcination. During calcination, the heating rate is 5°C / min, the temperature is raised to 200°C, and the t...
Embodiment 3
[0067] Ba 0.4 Sr 0.6 Fe 0.5 co 0.5 o 3-δ Preparation of photocatalyst:
[0068] Weigh 0.04mol Ba(NO 3 ) 2 , 0.06mol Sr(NO 3 ) 2 , 0.04mol Co(NO 3 ) 2 ·6H 2 O, 0.04mol Fe(NO 3 ) 3 9H 2 O, 0.13mol citric acid, put into the beaker, then add 400ml of distilled water, stir until dissolved, cover the opening of the beaker with a watch glass, seal it, then place the beaker on a constant temperature magnetic stirrer for heating and stirring, and heat to 94°C , stirred at constant temperature for 4 hours, opened the opening of the beaker, continued to heat until the water evaporated to form a colloid, placed the colloid in a blast drying oven for 21 hours and kept it dry for 21 hours at a temperature of 130°C;
[0069] (2) Ba 0.4 Sr 0.6 Fe 0.5 co 0.5 o 3-δ Calcination of photocatalyst: Take out the dried sample, grind it finely, put it into an alumina crucible, and carry out calcination. During calcination, the heating rate is 5°C / min, the temperature is raised to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com