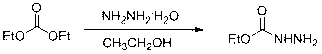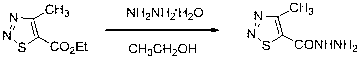2-(4-methyl-1,2,3-thiadiazole)-5-(substituent)-1,3,4-oxa(thia)diazole derivative and application thereof
A technology of azole derivatives and thiadiazoles, applied in the field of 2--5--1,3,4-oxadiazole derivatives, which can solve problems such as uneven color, reduced quality, and poor smoke taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] Example :Synthesis of target compound
[0045] Synthesis of 2-(4-methyl-1,2,3-thiadiazole)-5-(substituent)-1,3,4-oxadiazole derivatives
[0046] Synthesis of intermediate ethyl carbazate
[0047]
[0048] Put diethyl carbonate (11.8g, 0.1mol) and 80% hydrazine hydrate (5.9g, 0.095 mol) into a 100 mL three-necked flask, stir at room temperature (23℃) for 4h, TCL followed the reaction (iodine color, V petroleum Ether: V ethyl acetate=3:1). The ethanol and water were removed under reduced pressure, and colorless crystals were precipitated. The product was obtained with a pumping rate of 9g, with a melting point of 44~45℃ (45~46℃, Fan, Z. J., et al, 2009), and a yield of 87%.
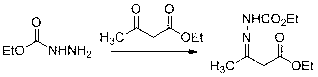
[0049] Synthesis of condensation intermediates
[0050]
[0051] Put ethyl acetoacetate (6.5g, 0.05 mol) and dichloromethane (35 mL) into a 100 mL three-necked flask, and dissolve ethyl carbazate (5.2g, 0.05 mol) in dichloromethane (17 mL) Then slowly drip into the three-necked bottle, the dripping is comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com