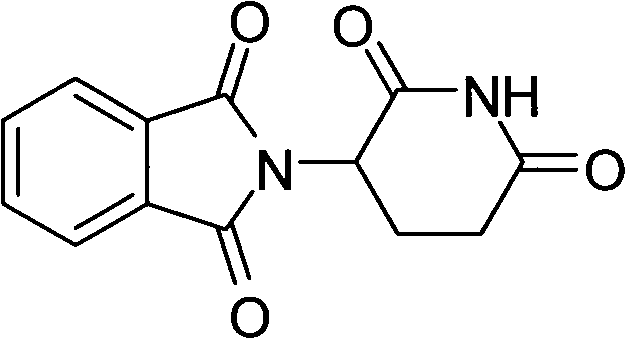
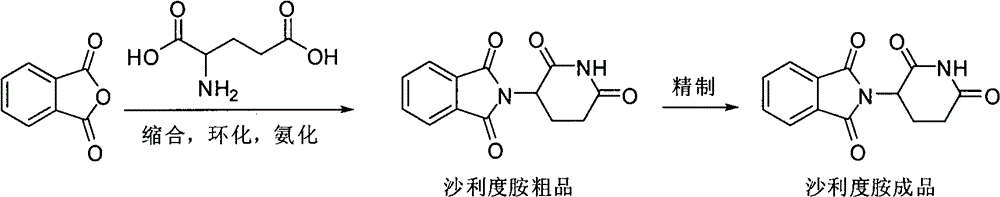
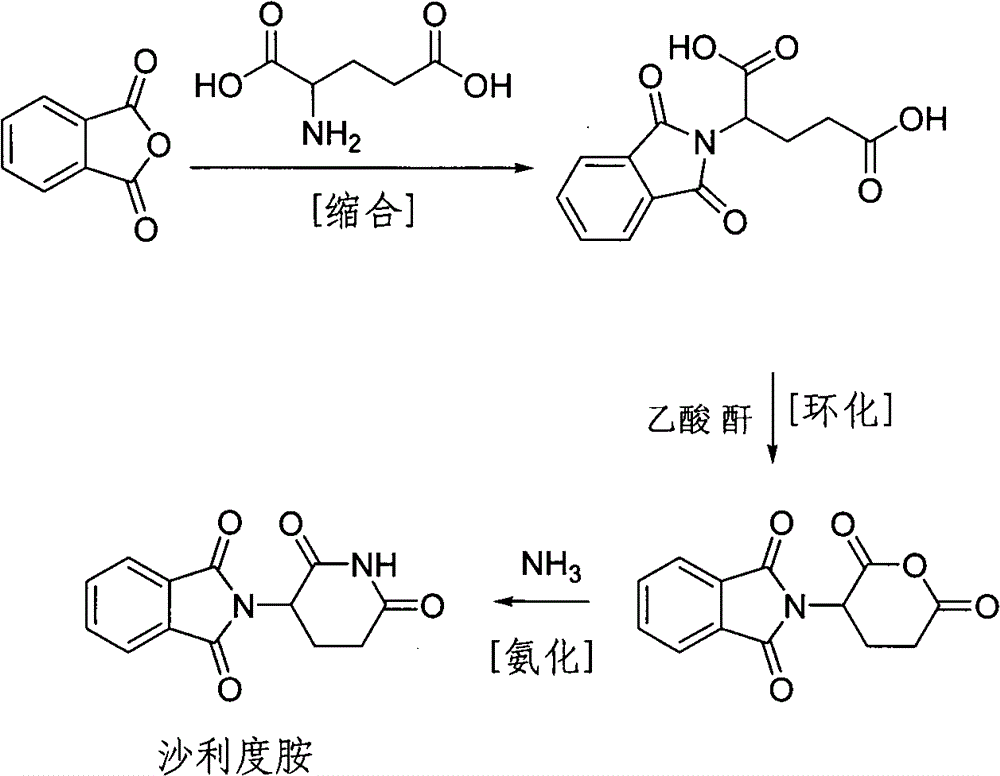
Synthetic method of medicine for treating leprosy
A technology for treating drugs and leprosy, which is applied in the field of medicine, and can solve problems such as long process routes, heavy environmental pollution, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Pump 100kg of N, N-dimethylformamide into the reactor, add 50kg of glutamic acid and 50kg of phthalic anhydride under stirring, then heat, react at 95-100°C for 3 hours, and then distill out N , N-dimethylformamide, cooling and adding 100kg of acetic anhydride, heating to 105-110°C for 1 hour reaction, decompressing to remove excess anhydride, heating the reaction to 230°C, slowly feeding ammonia gas under stirring, and reacting After that, discharge the material while it is hot, put it into a reaction pot with 300kg of water in advance, cool to room temperature, and filter to obtain the crude thalidomide, put the crude thalidomide into a tank with 300L dimethyl sulfoxide In the refining pot, add activated carbon, reflux for 1 hour, filter into the crystallization pot, cool to room temperature, filter, and dry to obtain 70.2 kg of thalidomide finished product, with a yield of 80.6%, a melting point of 274-279°C, and a purity of 99.8%. 1 HNMR (DMSO-d 6 ): 11.12(s, 1H), ...
Embodiment 2
[0022] Draw 100kg of N-methylpyrrolidone into the reactor, add 50kg of glutamic acid and 50kg of phthalic anhydride under stirring, then heat, react at 125-130°C for 1 hour, and then distill out N-methylpyrrolidone under reduced pressure , add 100kg of acetic anhydride after cooling, heat to 80-85°C to react for 3 hours, evaporate the excess anhydride under reduced pressure, heat the reaction to 180°C, slowly feed ammonia gas under stirring, and monitor the reaction by thin-layer chromatography. Discharge the material while it is hot, put it into a reaction pot with 300kg of water in advance, cool to room temperature, filter to obtain the crude thalidomide, put the crude thalidomide into the refining pot with 300L dimethyl sulfoxide , add activated carbon, reflux for 1 hour, filter into a crystallization pot, cool to room temperature, filter, and dry to obtain 72.5 kg of thalidomide finished product, with a yield of 83.2%, a melting point of 274-275°C, and a purity of 99.8%.
Embodiment 3
[0024] Pump 100kg of N, N-dimethylformamide into the reactor, add 50kg of glutamic acid and 50kg of phthalic anhydride under stirring, then heat, react at 80-85°C for 5 hours, and then distill out N , N-dimethylformamide, cooling and adding 100kg of acetic anhydride, heating to 115-120 ° C for 1 hour, decompression to remove excess anhydride, heating the reaction to 230 ° C, and slowly feeding ammonia gas under stirring, the reaction After completion, discharge the material while it is hot, put it into a reaction pot with 300kg of water in advance, cool to room temperature, filter to obtain the crude thalidomide, put the crude thalidomide into a refining pot with 300L of isopropanol Inside, add activated carbon, reflux for 1 hour, filter into a crystallization pot, cool to room temperature, filter, and dry to obtain 75.1 kg of thalidomide finished product, with a yield of 86.2%, a melting point of 273-275°C, and a purity of 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com