Chitosan derivative with cross-linking polymerization and containing drug ligand
A chitosan derivative, cross-linking polymerization technology, applied in pharmaceutical science, instruments, glasses/goggles, etc., can solve the lack of drug interaction ligands, chitosan cannot be cross-linked, and it is difficult to achieve drug control Release and other issues, to achieve the effects of great social and economic benefits, good transparency, and good processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
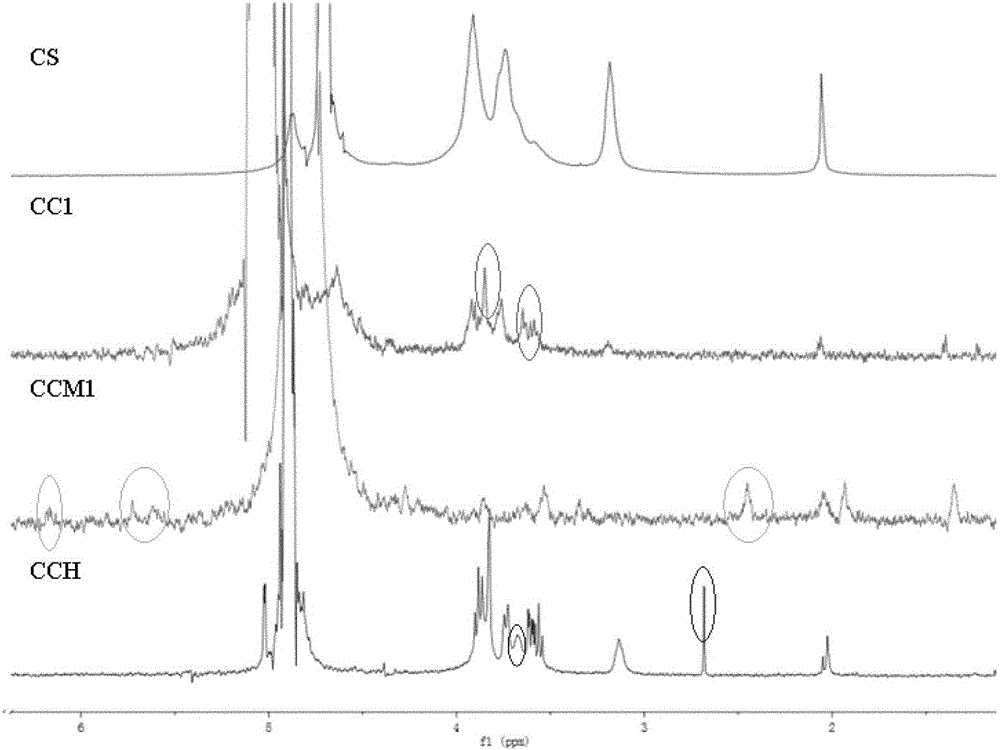
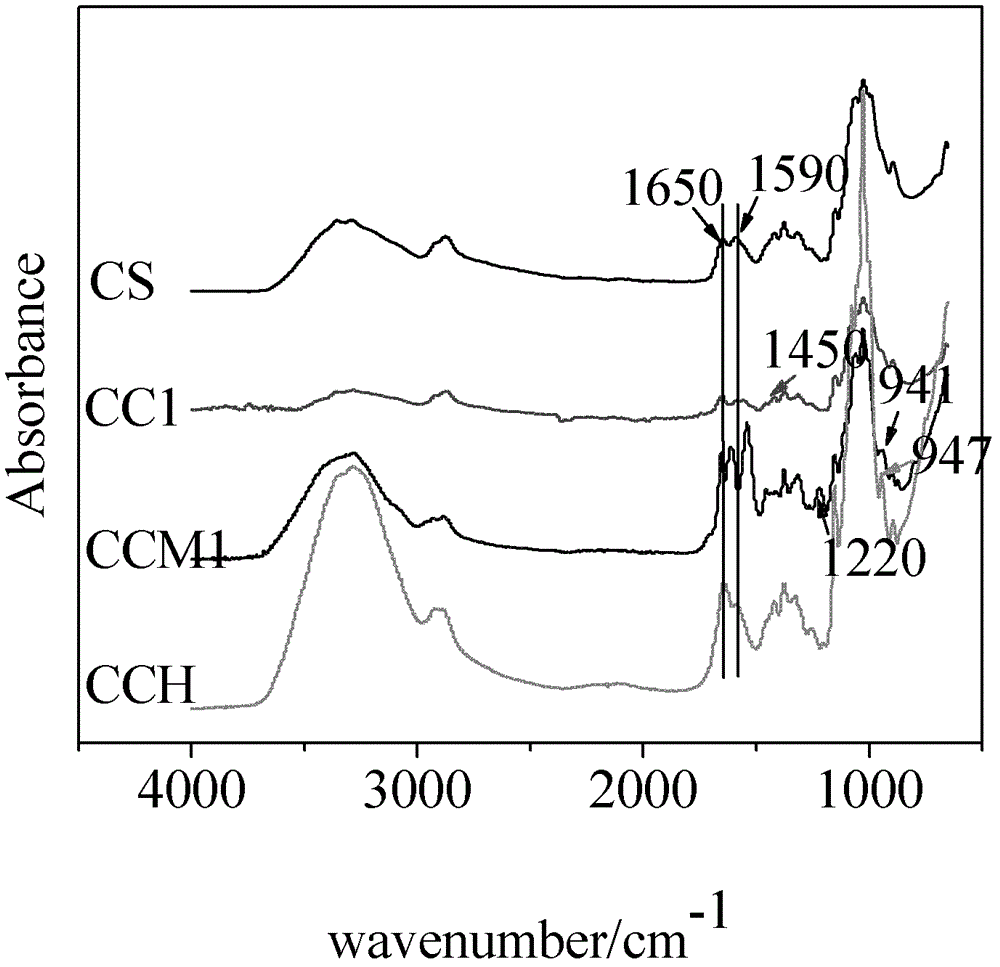
[0041] Add 3.0g of β-CD, 10.0mL of dimethyl sulfoxide (DMSO), and 10.0mL of isopropanol to the reactor in sequence, add 0.21mL of epichlorohydrin dropwise under magnetic stirring, and immediately add dropwise 0.55mol / L NaOH9 .15mL solution, after stirring for 4.5h, add 1% (m / m) CS / H 2 O50mL, continue to stir for 4.5h, dialyze for 72h, and freeze-dry to obtain the grafted product CC1; add it to the reactor, dissolve it in 100ml deionized water, and stir until completely dissolved. Add triethylamine 2.2mL, MAA 2.2mL and tetrabutylammonium bromide 2.2g to the previous CC1 / H 2 O solution, stirred at a constant temperature of 60 °C for 1 h. After the reaction, the solution was cooled to room temperature, dialyzed for about 72h, and freeze-dried to obtain the grafted product CCM1 (the Fourier transform infrared spectrogram of the relevant grafted product of this example is shown in the appendix figure 1 and NMR spectra are attached figure 2 ).
Embodiment 2
[0043] Add 1.5g of β-CD, 10.0mL of dimethyl sulfoxide (DMSO), 10.0mL of isopropanol to the reactor in sequence, add 0.21mL of epichlorohydrin dropwise under magnetic stirring, and immediately add dropwise 0.55mol / L NaOH9 .15mL solution, after stirring for 4.5h, add 1% (m / m) CS / H 2 O50mL, continued to stir for 4.5h, dialyzed for 72h, and freeze-dried to obtain the grafted product CC2; it was added to the reactor, dissolved in 100ml of deionized water, and stirred until completely dissolved. Add 2.2 mL of triethylamine, 2.2 mL of MAA and 2.2 g of tetrabutylammonium bromide to the previous CC2 / H 2 O solution, stirred at a constant temperature of 60 °C for 1 h. After the reaction, the solution was cooled to room temperature, dialyzed for about 72 hours, and freeze-dried to obtain the grafted product CCM2. Its infrared spectrum data: CC2, 1590cm -1 The primary amine peak is obviously weaker than 1650cm -1 Amide peak, 1450cm -1 The absorption peak of secondary amine (-NH-R) is ...
Embodiment 3
[0045] Add 6.0g of β-CD, 10.0mL of dimethyl sulfoxide (DMSO), and 10.0mL of isopropanol to the reactor in sequence, add 0.21mL of epichlorohydrin dropwise under magnetic stirring, and immediately add dropwise 0.55mol / L NaOH9 .15mL solution, after stirring for 4.5h, add 1% (m / m) CS / H 2 O50mL, continue to stir for 4.5h, dialyze for 72h, and freeze-dry to obtain the grafted product CC3; add it to the reactor, dissolve it in 100ml deionized water, and stir until completely dissolved. Add 2.2mL of triethylamine, 2.2mL of MAA and 2.2g of tetrabutylammonium bromide to the previous CC3 / H 2 O solution, stirred at a constant temperature of 60 °C for 1 h. After the reaction, the solution was cooled to room temperature, dialyzed for about 72 hours, and freeze-dried to obtain the grafted product CCM3. Its infrared spectrum data: CC3, 1590cm -1 The primary amine peak is obviously weaker than 1650cm -1 Amide peak, 1450cm -1 The absorption peak of secondary amine (-NH-R) is attributed to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com