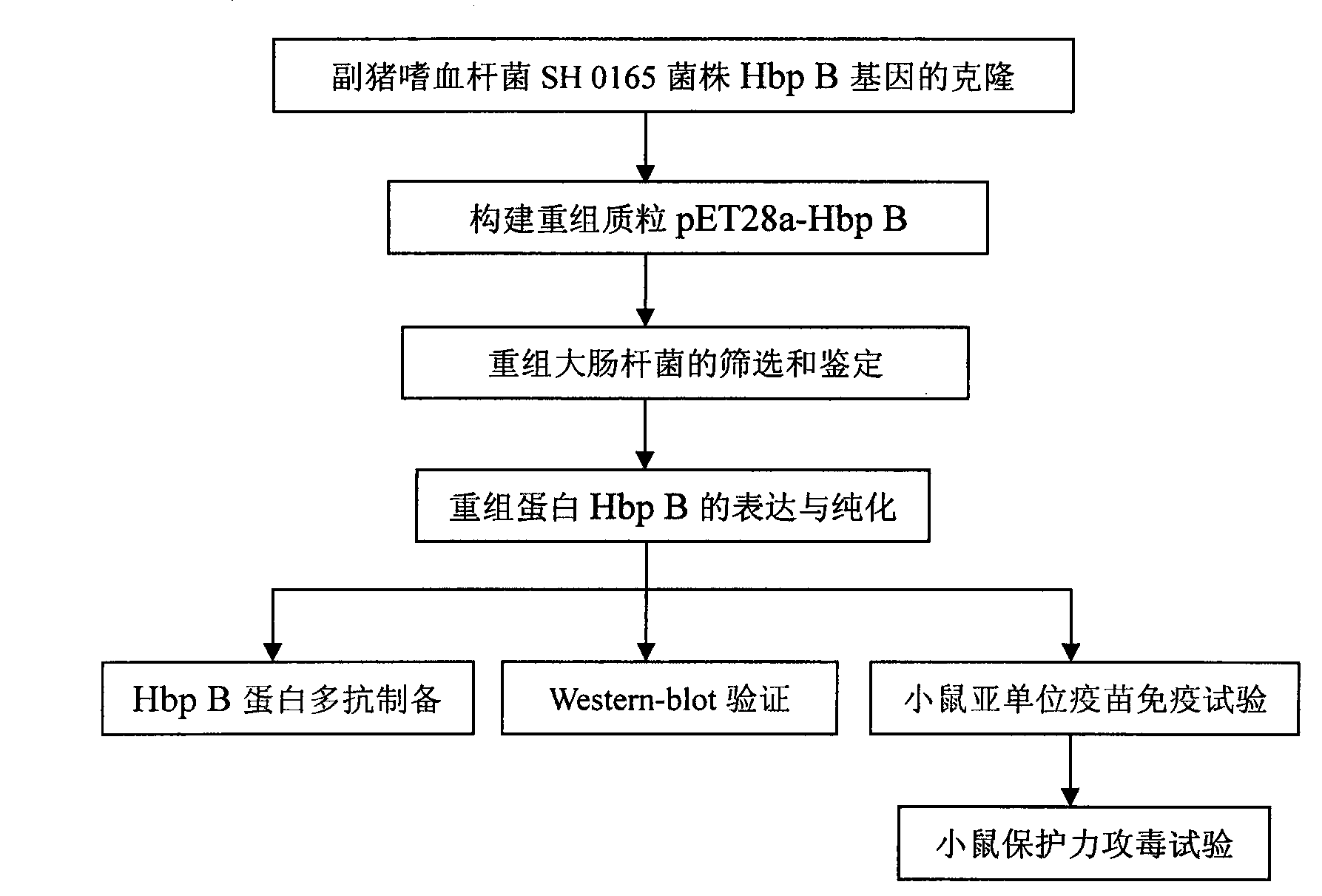
Immune protective antigen of haemophilus parasuis
A technology of Haemophilus suis and antigenic protein, which is applied in the field of subunit vaccine preparation of animal infectious diseases, can solve the problems affecting the control effect of commercial vaccines, Haemophilus parasuis is not, is not, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Cloning and expression of Haemophilus parasuis recombinant antigenic protein Hbp B
[0022] a material
[0023] 1 Plasmids and strains
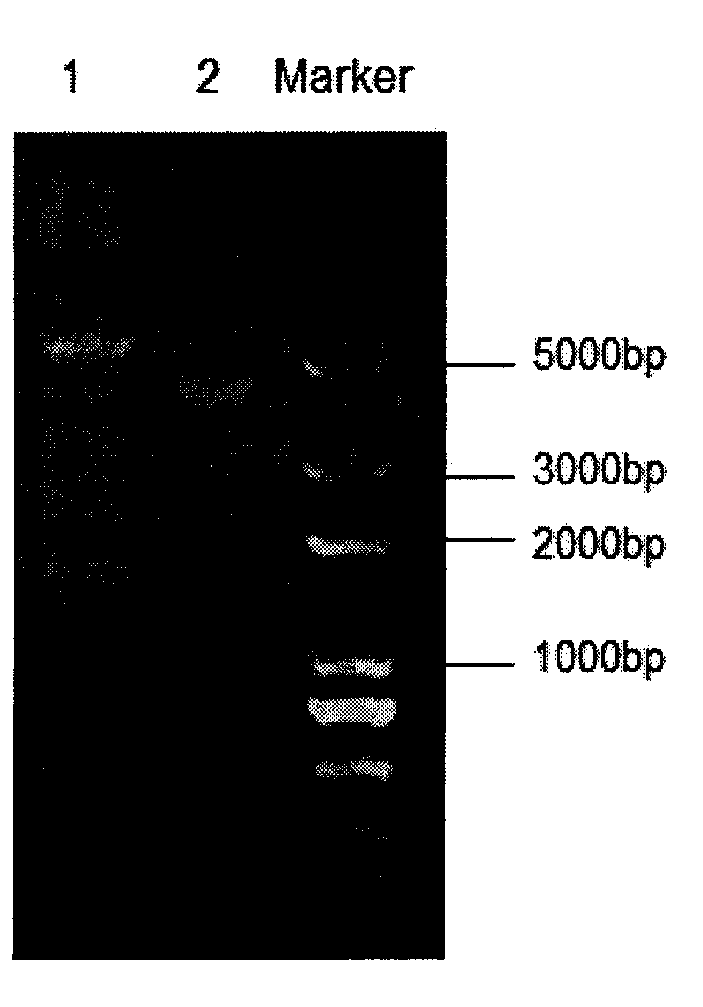
[0024] The pET-28a (+) plasmid used in the present invention is purchased from Novagen, Germany, and the pET-28a (+) plasmid map is as follows Figure 7 shown. Competent cells Escherichia coli DH5α and BL 21 (DE 3) are products of Beijing Quanshijin Biotechnology Co., Ltd.
[0025] The Haemophilus parasuis bacterial strain used in the present invention is the HPS SH0165 bacterial strain and screens for the State Key Laboratory of Agricultural Microbiology of Huazhong Agricultural University (referring to: Cai Xuwang, the research on the separation and identification of Haemophilus parasuis and the diagnostic method and inactivated vaccine, 2006 6 Month, Ph.D. Dissertation of Huazhong Agricultural University, National Library of China, National Digital Library of China http: / / res4.nlc.gov.cn / home / search.trs?method=showDeta...
Embodiment 2
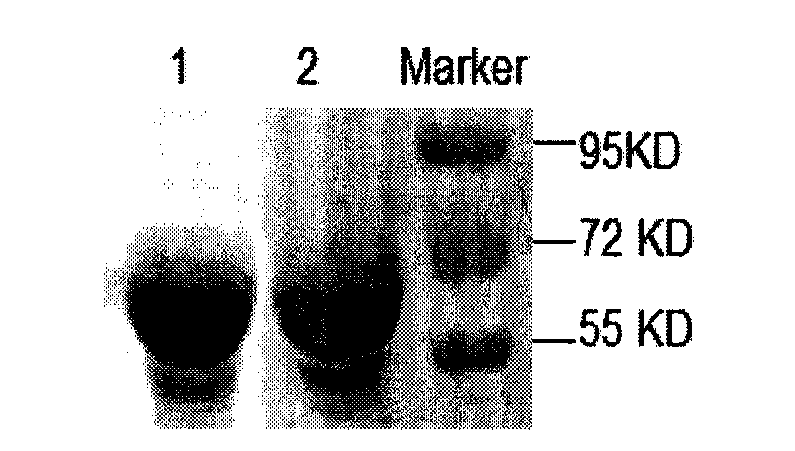
[0113] Example 2: Characteristic Analysis of Recombinant Antigen Protein
[0114] 1 Analysis of the characteristics of the recombinant antigenic protein by Western-blot method
[0115] The recombinant antigen protein Hbp B refolded and purified in Example 2 was subjected to SDS-PAGE gel electrophoresis according to conventional methods. Subsequent steps are as follows:
[0116] 1) Transfer membrane: Cut out 6 pieces of Whatman 3M filter paper and 1 piece of nitrocellulose membrane (NC membrane). The size of the filter paper and NC membrane should be completely equal to or slightly smaller than the gel, and mark the corner of the NC membrane with a pencil , determine the relative direction of the membrane and the gel after transfer; soak the NC membrane in purified water for 5 min; add a small amount of transfer buffer to another shallow tray, and soak 6 layers of Whatman 3M filter paper in it. Then install the transfer electrophoresis tank according to the following steps: l...
Embodiment 3
[0138] Embodiment 3: Recombinant antigen protein mouse immune protection test
[0139] 1 Mass expression and purification of recombinant proteins
[0140] A single colony containing the recombinant expression strain was inoculated into 10 mL of LB liquid medium containing 0.25 μg / mL kanamycin, and cultured on a shaker at 37°C. Inoculate the cultured bacteria solution into 1L of fresh LB liquid medium containing 2.5μg / mL kanamycin, shake and culture at 37°C for about 3h, until the OD 600 When it reaches 0.6-0.8, add IPTG to a final concentration of 0.8mmol / L, continue to induce for 3h, and then collect the bacteria. See Example 2 for the specific purification process of the recombinant protein.
[0141] 2 Determination of recombinant protein concentration (conventional Bradford method)
[0142] See embodiment 2 for specific operations.
[0143] 3 Preparation of recombinant antigenic protein vaccine and immunization method
[0144] 1) Preparation of recombinant antigen protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com