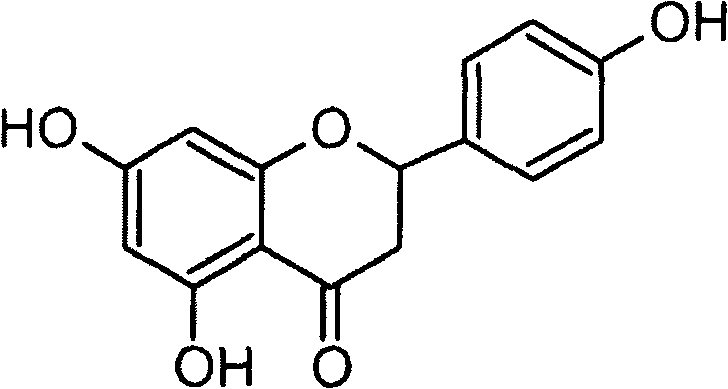
Application of naringenin in preparation of medicine for curing pneumonia
A technology of naringenin and pneumonia, applied in the field of medicine, can solve the problems of reduced efficacy, high incidence of pneumonia and high mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
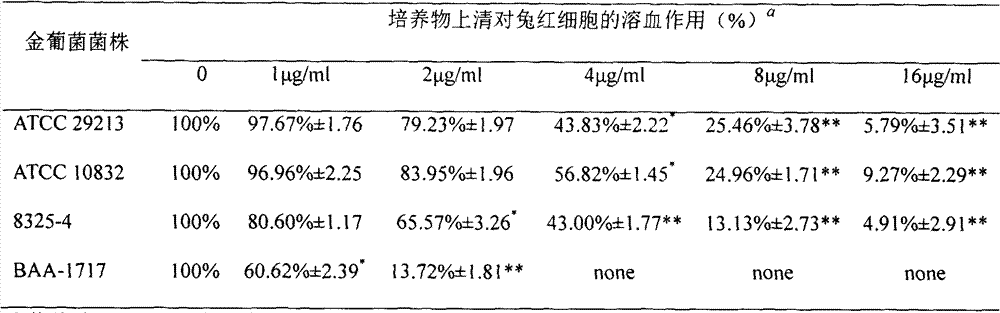
[0007] 1. Hemolysis test
[0008] Staphylococcus aureus culture Then co-culture with different concentrations of naringenin until the late logarithmic growth period, harvest the bacterial liquid and centrifuge to get the supernatant. The culture supernatants of different drug concentrations were mixed with diluted rabbit defibrotic red blood cells, and incubated at 37°C for 10-20 minutes. Centrifuge to measure the absorbance of the supernatant.
[0009] The percentage of hemolysis after adding different concentrations of naringenin is shown in Table 1:
[0010] Table 1. Supernatant hemolysis of ATCC 29213, ATCC 10832, 8325-4 and BAA-1717 in cultures without treatment and with different concentrations of naringenin
[0011]
[0012] a The hemolysis effect of the culture supernatant of Staphylococcus aureus in the drug-untreated group on rabbit erythrocytes was set as 100% hemolysis: none means that hemolysis was observed;
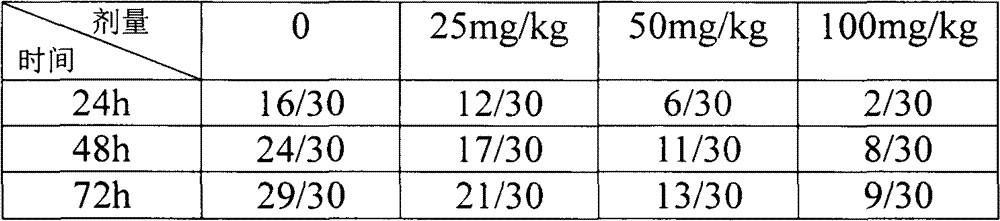
[0013] The t test was used to analyze the dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com