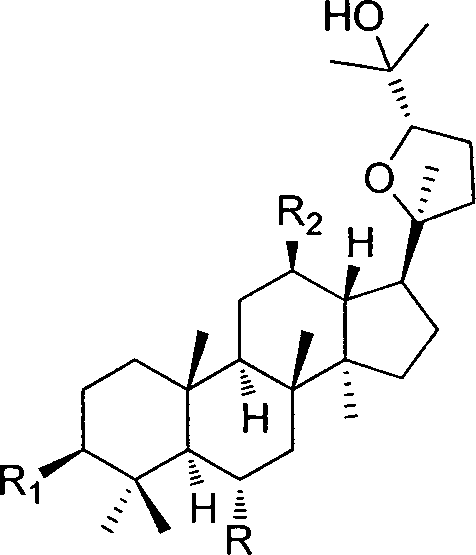
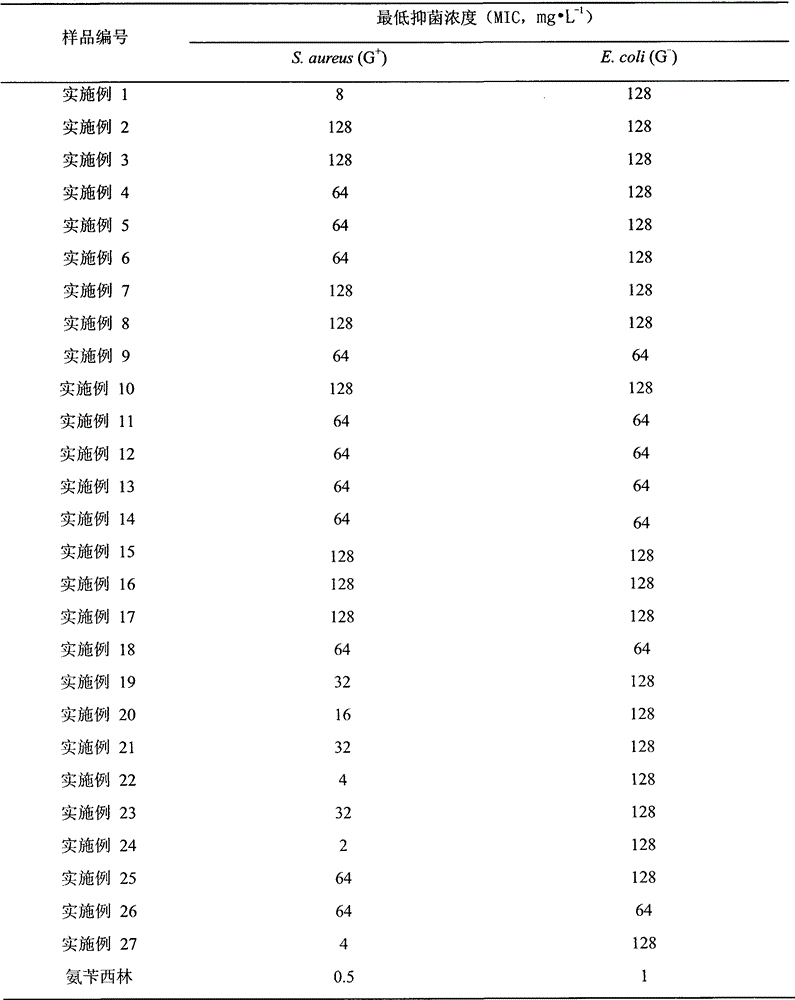(20S,24S)-ocotillol ginsenoside derivatives with antibacterial activity, and preparation method and application thereof
A technology of compounds and medicinal salts, applied in the field of new -ocotillol ginsenoside derivatives, which can solve the problems of low water solubility and limited application of ginsenosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] (20S, 24S)-epoxydammarane-3β, 12β, 25-triol
[0074] Dissolve 20(S)-protopanaxadiol (500mg, 1.08mmol) in anhydrous pyridine (3ml), add DMAP (20mg, 0.16mmol), stir well and slowly add acetic anhydride (0.42ml, 4.43mmol) dropwise , stirred at room temperature for 12h. Dilute with ethyl acetate (20ml), wash with 10% hydrochloric acid until acidic, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography (petroleum ether: ethyl acetate = 10:1) to give white Solid A-1 (508 mg, 85%).
[0075] The A-1 (208mg, 0.38mmol) obtained above was dissolved in anhydrous dichloromethane (6ml), and m-chloroperoxybenzoic acid (185mg, 75%, 0.16mmol) was slowly added dropwise under pre-cooling in an ice-salt bath. Dichloromethane (5ml) solution, half an hour after the dropwise addition was completed, it was raised to room temperature and stirred for 2h. Add isopropanol (0.1ml), continue to stir for one hour, add saturate...
Embodiment 2
[0078] (20S, 24S)-epoxydammarane-3β, 6α, 12β, 25-tetrol
[0079] Using 20(S)-protopanaxatriol as raw material, the reference (20S, 24S)-epoxydammarane-3β, 12β, 25-triol synthesis method was implemented to obtain a white solid (58 mg, 48.3%). 1 H NMR (CDCl 3 , 300MHz) δ4.09-4.15 (dd, J = 11.0Hz, 7.1 Hz, 1H), 3.84-3.90 (dd, J = 10.4Hz, 5.1 Hz, 1H), 3.48-3.57 (td, J = 10.2Hz, 4.6Hz, 1H), 3.15-3.21(dd, J=11.1Hz, 5.0 Hz, 1H), 2.21-2.29(td, J=10.6Hz, 4.1Hz, 1H), 1.32(s, 3H), 1.27(s , 3H), 1.23(s, 3H), 1.10(s, 3H), 1.09(s, 3H), 0.98(s, 3H), 0.94(s, 6H). MS (ESI) m / z: 493.3 [M+H ] +.
Embodiment 3
[0081] (20S, 24S)-epoxydammarane-12β, 25-diol-3-one
[0082] Compound 2 (40mg, 0.08mmol) was dissolved in anhydrous dichloromethane (3ml), PCC (pyridinium chlorochromate, 36mg, 0.17mmol) was added, and stirred at room temperature for 3 hours. The solvent was removed under reduced pressure, dissolved in ethyl acetate, extracted, the organic layer was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography (petroleum ether:ethyl acetate=10:1) to obtain a white solid ( 23 mg, 58%). 1 H NMR (CDCl 3 , 500 MHz) δ3.87-3.90 (dd, J = 10.5Hz, 5.0 Hz, 1H), 3.52-3.57 (td, J = 10.0Hz, 4.5Hz, 1H), 2.49-2.54 (m, 1H), 2.42 -2.48(ddd, J=11.0Hz, 8.0Hz, 3.5Hz, 1H), 2.24-2.29(td, J=10.5Hz, 4.5Hz, 1H), 1.28(s, 3H), 1.23(s, 3H), 1.11(s, 3H), 1.08(s, 3H), 1.053(s, 3H), 1.046(s, 3H), 0.96(s, 3H), 0.93(s, 3H). MS (ESI) m / z: 475.3 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com