Application of lapachol to preparation of anti-human-immunodeficiency-virus-1 (HIV-1) medicine
A technology of HIV-1 and Lappaol, which is applied in the field of medicine and biology, can solve problems such as the report of no compound Lappaol, and achieve the effect of solving the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
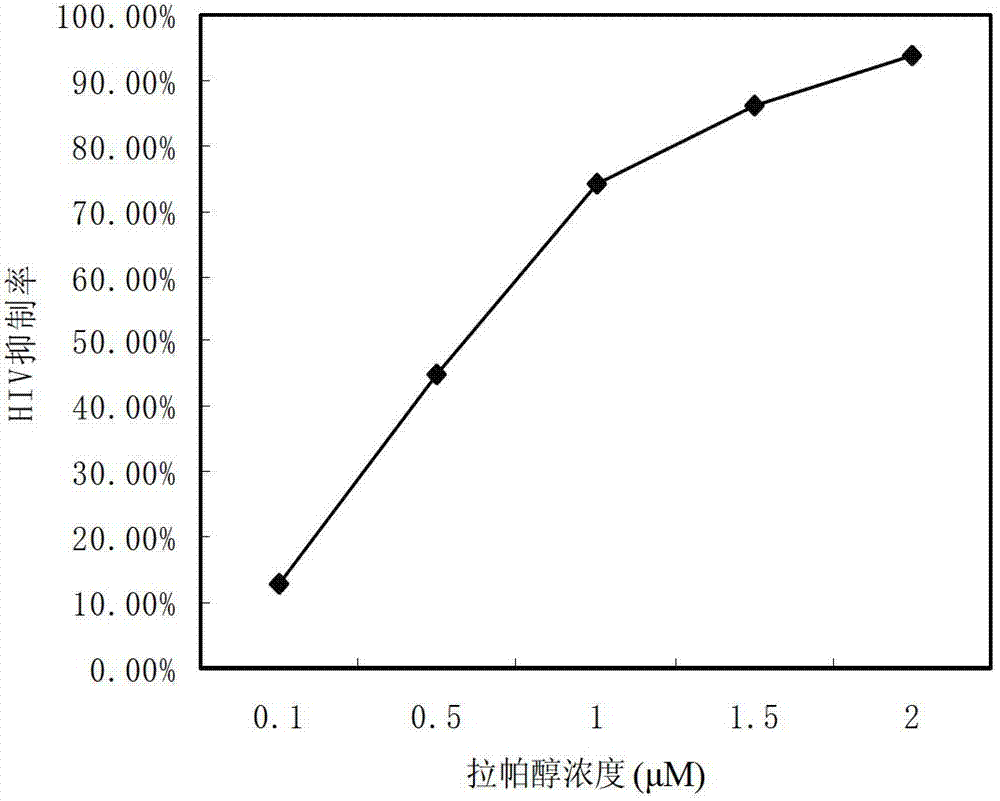
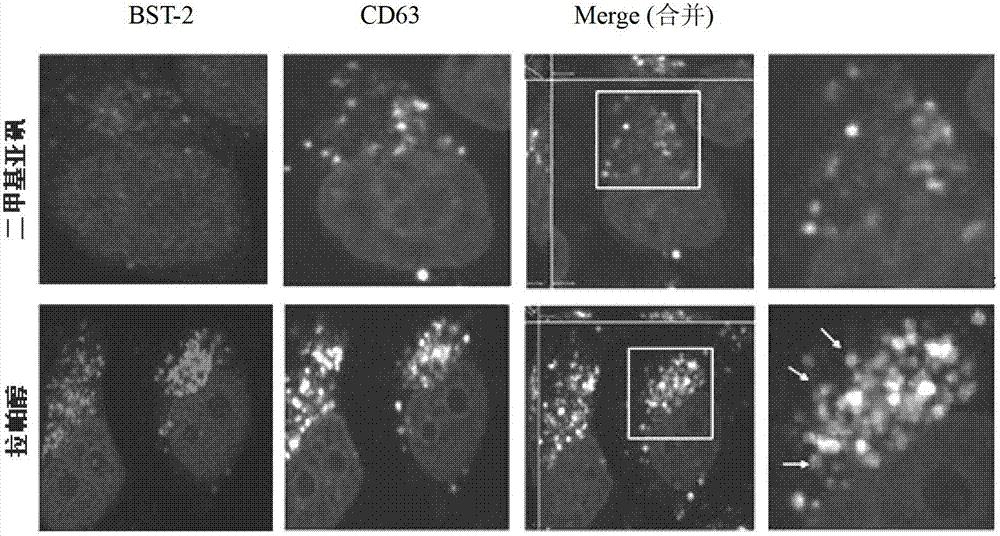
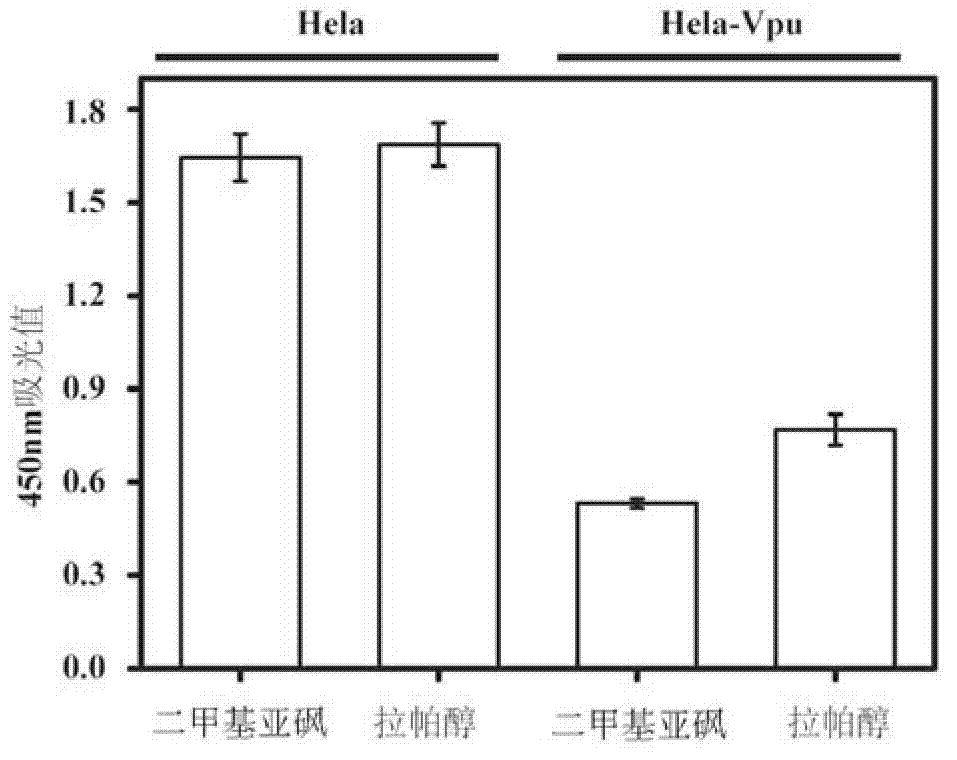
[0031] Example 1 Compound Lappaol inhibits Vpu down-regulation of BST-2 on the cell surface
[0032] Hela-Vpu cells stably expressing Vpu protein were constructed and screened by Cen Shan et al. (Cen Shan et al.. Screening of antiviral drugs that antagonize the activity of Vpu degrading BST-2. Patent application number: 201010123002.1), and BST-2 on its surface was screened by Vpu Down-regulated, lower than normal Hela.
[0033] Seed Hela cells and Hela-Vpu cells in a 96-well plate with 1.0×10 cells per well 4 indivual. At 37°C, 5% CO 2 After culturing in the incubator for 24 hours, dimethyl sulfoxide (DMSO) and Lappaol were added to treat them respectively. After continuing to culture for 24 hours, the expression level of BST-2 on the cell membrane surface was detected by Cell-ELISA. The detection process is as follows: suck off the old culture medium, wash twice with PBS, 2 min each time. Add 100 μl of 4% paraformaldehyde to each well and fix at room temperature for 20 m...
Embodiment 2
[0035] Effect of Example 2 Compound Lappaol on the Transcription of BST-2mRNA
[0036] In order to determine the effect of the compound lapachol on BST-2 mRNA transcription, Hela-Vpu cells were seeded in 6-well plates with 2.0×10 cells per well. 5 indivual. 37°C, 5% CO 2 After culturing in the incubator for 24 hours, DMSO and Lappaol were added for treatment, and after culturing for another 24 hours, total cellular RNA was extracted according to the instructions of Invitrogen's TRIZOL. After the concentration was determined, 2 μg of total RNA was taken, and the RNA was reverse-transcribed into cDNA using M-MLV reverse transcriptase and random primers.
[0037] cDNA amplification reaction system: SsoFast EvaGreen Supermix 10 μl, forward primer (10 μM) 1 μl, reverse primer (10 μM) 1 μl, template cDNA 0.5 μl, RNase-free water 7.5 μl. The reaction condition is 95°C, 30 sec; then 40 cycles, each cycle condition is 95°C, 5 sec; 60°C, 20 sec. The melting curve is 65°C-95°C, conti...
Embodiment 3
[0043] The influence of the Vpu expression level of embodiment 3 compound Lappaol
[0044] Hela-Vpu cells were seeded in a 6-well plate with 2.0×10 cells per well 5 indivual. 37°C, 5% CO 2 After culturing in the incubator for 24 hours, add dimethyl sulfoxide, concanavalin A, and lapachol for treatment, and continue culturing for 24 hours. Scrape the cells in the 6-well plate, resuspend in 1ml pre-cooled PBS, 2×10 3 Collect the cells by centrifugation at g for 5 minutes, remove the supernatant, add 50 μl of pre-cooled PBS to resuspend, then add 50 μl of 2× loading buffer to lyse the cells, bathe in boiling water for 10 minutes, and vortex every 3 minutes. SDS-PAGE separated protein samples. After transferring to PVDF membrane, it was incubated with primary antibody and secondary antibody successively, and then developed by ECL. The primary antibody uses the following concentrations of rabbit polyclonal BST-2 antibody (1:5000), rabbit polyclonal Vpu antibody (1:1000), mouse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com